1. There are multiple oligomeric forms of G protein-coupled receptors (GPCRs).
G protein-coupled receptors (GPCRs) are a crucial class of receptor proteins located on the surface of cells. In the human genome, genes encoding GPCRs account for over 2%, comprising more than 800 members, making them the largest class of cell membrane proteins[1]. GPCRs participate in regulating various cellular responses, and signaling pathways associated with GPCRs are implicated in the pathophysiology of numerous diseases, ranging from metabolic, immune, and neurodegenerative disorders to cancer and infectious diseases. Many GPCRs play pivotal roles and serve as important targets for drug development[2].
GPCRs are primarily classified into three major families: A, B, and C. Traditional concepts suggest that GPCRs primarily function as monomers. Existing structural data also indicate that monomeric GPCRs can simultaneously bind agonists and G proteins. Through techniques such as fluorescence resonance energy transfer (FRET), it has been discovered that many GPCRs in the A/B family may undergo homodimerization, such as β2AR, AT1R, 5HT2C, and D2R receptors[3, 4].
The C family of GPCRs mainly includes metabotropic glutamate (mGlu) receptors, comprising eight members, and serving as crucial drug targets for psychiatric disorders[5]. mGlu receptors are considered typical homodimers[5], with no current evidence indicating their functionality in monomeric form. In overexpression systems, different subunits of mGlu can also form 16 distinct heteromeric dimers[6](Figure1), exhibiting specific pharmacological properties inconsistent with homodimers. Due to the lack of effective tools, including antibodies that are specific, high-affinity, and capable of recognizing receptor spatial conformations [7](Figure2), direct evidence for the existence of mGlu heteromeric dimers in the brain has been elusive.
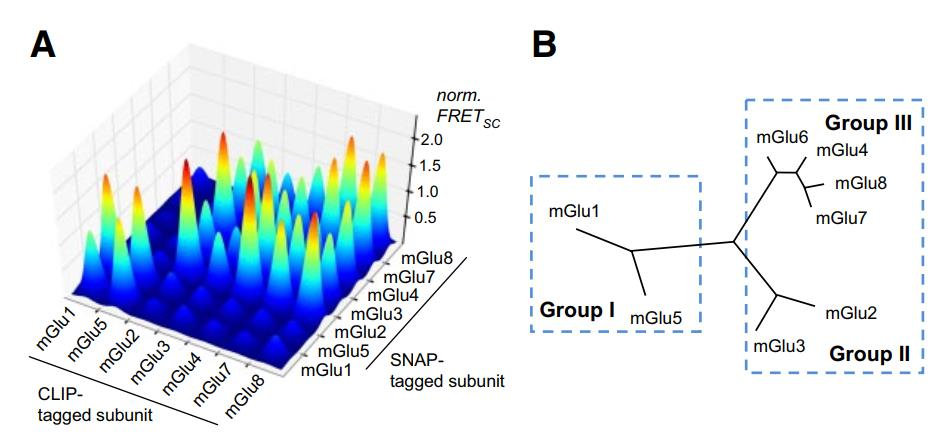
Figure 1 illustrates the occurrence of both homomeric and heteromeric dimerization of mGlu receptors in overexpression systems.
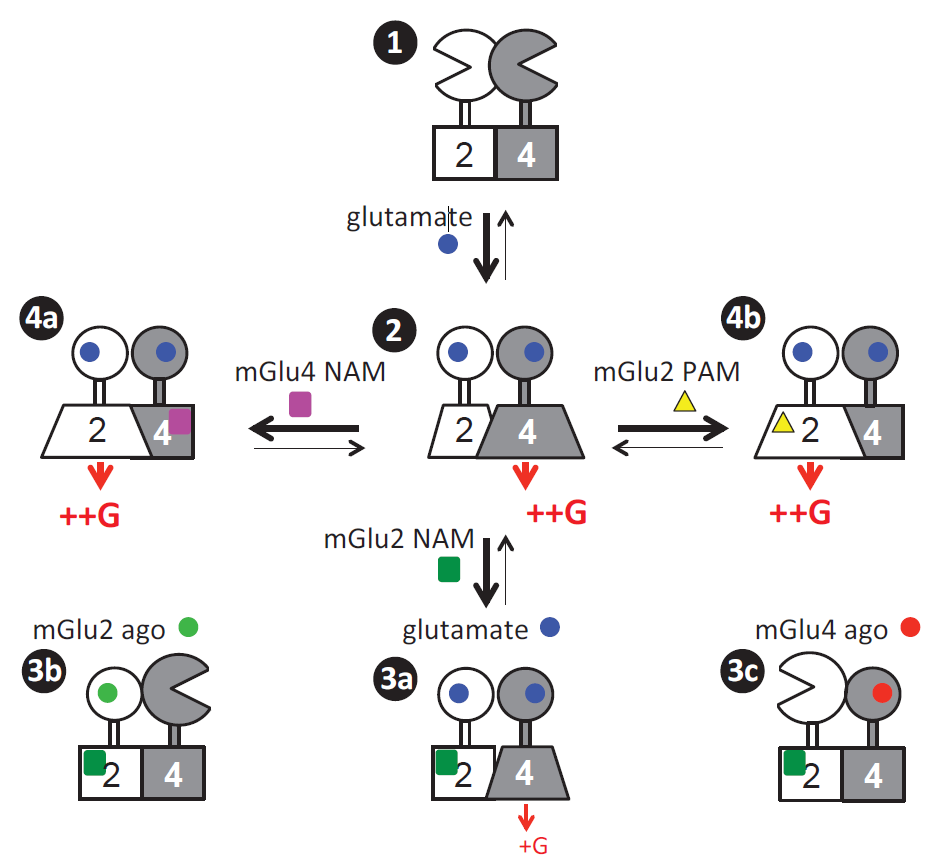
Figure 2 demonstrates the presence of various downstream signaling patterns activated by heteromeric dimers of mGlu2 and mGlu4.
2. Nanobodies provide potent tools for GPCR research.
In 1993, Belgian scientists first reported in the journal Nature the presence of naturally occurring antibodies lacking a light chain in the peripheral blood of llamas. These antibodies comprise solely a single variable domain of the heavy chain (VHH) along with two conventional CH2 and CH3 domains, yet exhibit exceptional stability. The individually cloned and expressed VHH structures demonstrate comparable structural stability to their original heavy chain antibodies and retain binding activity to antigens. Nanobodies represent the smallest units known to bind target antigens, with a molecular weight of approximately 15 kD, earning them the designation of "nanobodies" [8, 9].
Nanobodies, characterized by their small size, simple structure, enhanced tumor tissue penetration, ability to cross the blood-brain barrier, high affinity, low immunogenicity, good hydrophilicity, high stability, and ease of modification, have been widely employed in the development of therapeutic antibodies targeting members of the GPCR family. Numerous reports have demonstrated the successful development of nanobodies targeting various GPCRs. In addition to serving as the basis for developing a range of research tools to detect various GPCR parameters, nanobodies have also been shown to significantly modulate GPCR function, thus providing powerful tools for GPCR research [8].
GPCRs are the largest class of drug development targets, with over one-third of marketed drugs targeting GPCRs. However, the majority of these drugs are small molecules and peptides. Globally, only two antibody-based drugs targeting GPCRs have been approved for market, namely Mogamulizumab targeting CCR4 and Erenumab targeting the CGRP receptor. Nanobodies, as single-domain antibodies, provide a new direction for the development of antibody-based drugs targeting GPCRs.
3. Nanobodies can achieve high affinity, high specificity, and discrimination of different subtypes of mGlu receptors.
The sequence homogeneity among the eight members of mGlu receptors, particularly within subfamilies, poses significant challenges in effectively distinguishing them using small molecule ligands, antibodies, and other methods. Nanobodies, due to their specific recognition of target spatial conformations, can accurately distinguish and identify different subtypes. For instance, in a report published in NC in 2017, nanobodies such as DN1, developed by researchers, demonstrated the ability to precisely differentiate mGlu2 from other family members using the HTRF detection method. This represents the only method currently available besides distinguishing mGlu2 and mGlu3 subtypes based on gene sequences [10].
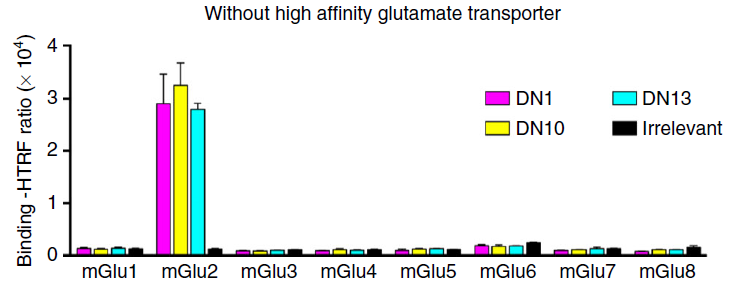
Nanobodies, owing to their high affinity and specificity, are poised to play a significant role in the recognition and detection of GPCRs and other areas.
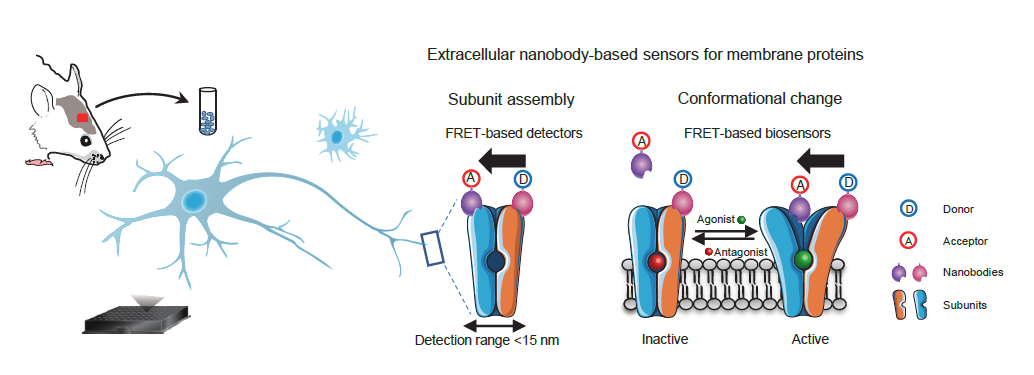
4. Developing biosensors based on nanobodies to detect the presence of endogenous homomeric and heteromeric dimers of mGlu receptors.
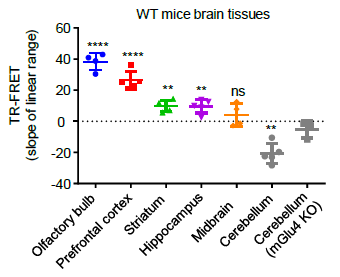
Researchers can detect the activation of both homomeric mGlu2 dimers and heteromeric mGlu2-4 dimers in brain samples, enabling comprehensive pharmacological analysis of the activity of endogenously expressed receptors in brain samples. Utilizing this novel approach, the study found that mGlu4 is expressed in most brain regions in the form of mGlu2-4 heteromeric dimers, while in the cerebellar region, mGlu4 exists only as homomeric dimers [11]. This discovery suggests a new strategy for drug development targeting mGlu2-4 heteromeric dimers, providing a theoretical basis for repurposing existing drugs targeting mGlu2 and mGlu4 as "old drugs for new uses". Additionally, this method enables rapid and high-throughput pharmacological detection of the activity of endogenous membrane receptor complexes in living tissues, addressing the longstanding challenge of detecting the expression distribution and activity of membrane protein complexes in human and animal tissues.

mGlu2 homodimer
mGlu2-4 heterdimer
Nanobodies play a crucial role in GPCR research, and in the future, they will assist scientists in uncovering more mysteries of GPCRs. Wuhan Nanobody Life Science & Technology (NBLST) leveraging its proprietary high-throughput antibody screening and detection platform, has accumulated years of experience in GPCR antibody screening. We are dedicated to providing more professional CRO services, including targeted GPCR nanobody screening, to support scientific advancement.
参考文献
[1] Dorsam R T, Gutkind J S. G-protein-coupled receptors and cancer[J]. Nat Rev Cancer, 2007,7(2):79-94.
[2] Hauser A S, Chavali S, Masuho I, et al. Pharmacogenomics of GPCR Drug Targets[J]. Cell, 2018,172(1-2):41-54.
[3] Cottet M, Albizu L, Comps-Agrar L, et al. Time resolved FRET strategy with fluorescent ligands to analyze receptor interactions in native tissues: application to GPCR oligomerization[J]. Methods in molecular biology (Clifton, N.J.), 2011,746:373.
[4] Hern J A, Baig A H, Mashanov G I, et al. Formation and dissociation of M1 muscarinic receptor dimers seen by total internal reflection fluorescence imaging of single molecules[J]. Proc Natl Acad Sci U S A, 2010,107(6):2693-2698.
[5] Pin J P, Bettler B. Organization and functions of mGlu and GABAB receptor complexes[J]. Nature, 2016,540(7631):60-68.
[6] Doumazane E, Scholler P, Zwier J M, et al. A new approach to analyze cell surface protein complexes reveals specific heterodimeric metabotropic glutamate receptors[J]. The FASEB Journal, 2011,25(1):66-77.
[7] Liu J, Zhang Z, Moreno-Delgado D, et al. Allosteric control of an asymmetric transduction in a G protein-coupled receptor heterodimer[J]. Elife, 2017,6.
[8] Manglik A, Kobilka B K, Steyaert J. Nanobodies to Study G Protein-Coupled Receptor Structure and Function[J]. Annu Rev Pharmacol Toxicol, 2017,57:19-37.
[9] Muyldermans S. Nanobodies: natural single-domain antibodies[J]. Annual review of biochemistry, 2013,82(1):775-797.
[10] Scholler P, Nevoltris D, de Bundel D, et al. Allosteric nanobodies uncover a role of hippocampal mGlu2 receptor homodimers in contextual fear consolidation[J]. Nat Commun, 2017,8(1):1967.
[11] Meng J, Xu C, Lafon P A, et al. Nanobody-based sensors reveal a high proportion of mGlu heterodimers in the brain[J]. Nat Chem Biol, 2022.







