Surface Plasmon Resonance (SPR) Analysis: Unlocking Molecular Interactions
With its high throughput, flexibility, and sensitivity, Surface Plasmon Resonance (SPR) analysis technology enables researchers to characterize the interactions between biomolecules in binding studies. It finds broad applications across various molecules, including ions, molecular fragments, small molecules, proteins, viruses, and more.
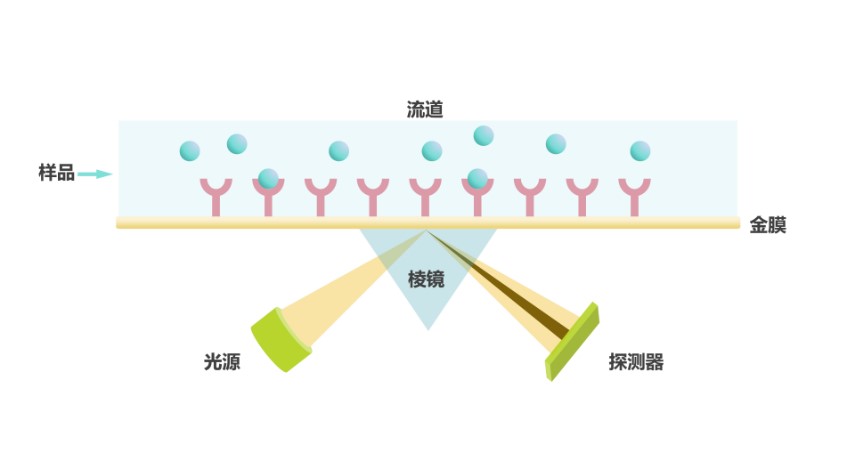
In simple terms, SPR technology detects the interactions between ligands and analytes on a biosensor chip, allowing us to explore the properties and structures of substances. This method enables real-time and precise analysis of various organic or inorganic substances in samples. It helps deduce important information such as binding constants (Ka), dissociation constants (Kd), affinity constants (KD), and binding kinetics between molecules. SPR technology significantly aids research in life sciences, medical diagnostics, and other fields.
Did you know that the discovery of SPR dates back over a century?
In 1902, scientist Wood observed the phenomenon of SPR during optical experiments and made simple records of it. It wasn't until 39 years later that another scientist named Fano provided a scientific explanation for the SPR phenomenon. Several decades passed, during which many scientists conducted experimental research, supplemented theories, and iterated on the technology. Finally, in 1990, Biacore AB developed the first commercial SPR instrument, opening up a much wider range of applications for this technology.
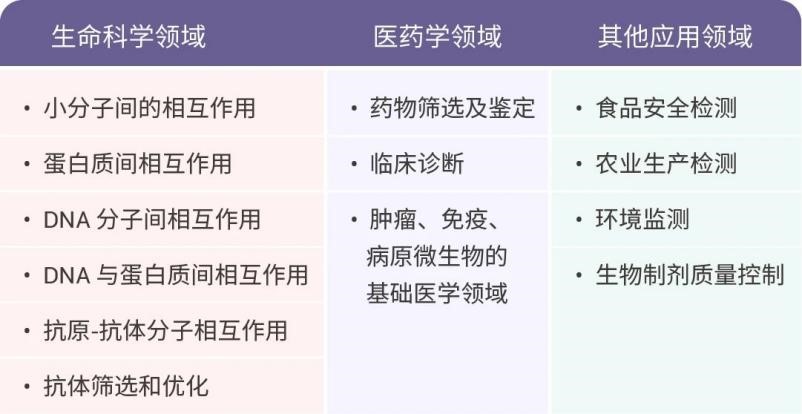
Since its first application in antigen-antibody interaction analysis by scientists like Liedberg in 1983 [1,2], SPR technology has been widely used in various fields including basic life sciences, pharmaceuticals, food, and environmental sciences. In recent years, advancements in both theoretical understanding and instrumentation have further expanded the application of SPR technology into areas such as small molecule interactions, proteomics, drug screening, and clinical diagnostics.
In 2020, SPR [3] detection was formally included as an important standard for detecting biological interactions in the "Chinese Pharmacopoeia." Over the past century, continuous research and development by numerous scientists have established SPR technology as an indispensable tool in contemporary life science research.
Characteristics of SPR Detection Technology:[3]
- Real-time detection, capable of dynamically monitoring the entire process of biomolecular interactions.
- Sample labeling is not required, preserving molecular activity.[4]
- Requires minimal sample amounts, typically only 100μg of protein per surface.
- Convenient and rapid detection process with high sensitivity.
- Wide range of applications.
- Provides high-quality analytical data.
- Can track and monitor the stability of immobilized ligands.[5]
Information provided by SPR Detection:
- Binding or non-binding (Yes or No).
- Specificity and selectivity of binding.
- Strength of binding between two molecules - Affinity.
- Rate of binding and dissociation, as well as stability of complexes - Kinetics.
- Thermodynamic characteristics such as temperature and binding.
- Detection of target molecule activity content - Concentration.
NBLST offers surface plasmon resonance (SPR) analysis technology services based on Biacore T200, suitable for various stages ranging from basic research, drug development to quality control. We provide customized services for diverse affinity analysis, including antigen-antibody, protein-protein, antibody-receptor, VLP protein-antibody, and protein-small molecule compound interactions.
Experimental Methods
Method 1 - Fixing IgG Antibody, Flowing Antigen Protein: Using a Protein A chip, IgG antibodies are fixed, and antigen proteins are flowed over, allowing for the natural conformation of the antigen-antibody binding, resulting in more accurate and reliable data.
Method 2 - Fixing Protein Ligands, Flowing Protein Analytes: Utilizing a CM5 chip, antigens are fixed via amino coupling, and IgG antibodies are flowed over. As long as the antigen protein remains stable, this method allows for repeated use and detection of interactions with multiple antibodies.
试验案例
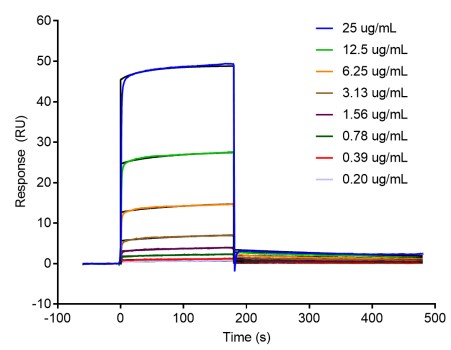
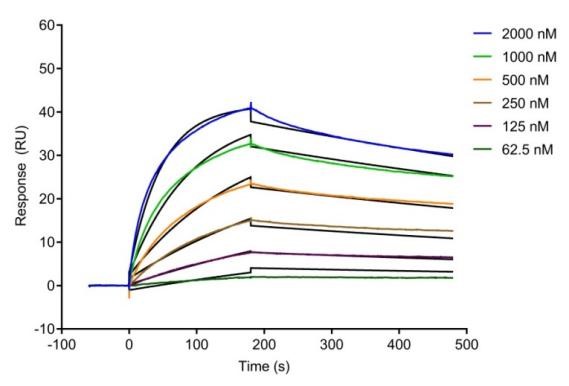
图1:分子与蛋白靶点结合检测 图2:纳米抗体与蛋白靶点结合检测
Advantages of NaBo SPR Detection Services:
(1) No molecular labeling required, saving labeling time and costs.
(2) Flexible experimental design, suitable for a wide range of applications.
(3) Based on the Biacore system, offering high sensitivity and stability.
(4) Comprehensive data acquisition including specificity, affinity, kinetic constants, etc.
(5) One-stop service solution from screening and validation to potency testing.
NBLST is committed to building an integrated experimental public service platform for production, education, and research. We aim to provide more professional and cost-effective experimental services for research institutions, pharmaceutical companies, industrial enterprises, and innovation teams.
If you have any questions or needs, please feel free to contact us at any time.
References:
[1] Liedberg B, et al. Surface plasmon resonance for gas detection and biosensing[J]. Sensors and Actuators, 1983, 4: 299-304.
[2] Nylander C, et al. Gas detection by means of surface plasmon resonance[J]. Sensors and Actuators, 1982, 3: 79-88.
[3] 程慧,黄朝峰。SPR生物传感器及其应用进展。中国生物工程杂志。2003,23(5):46-49
[4] Schasfoort RBM, et al. Trends in SPR Cytometry: Advances in Label-Free Detection of Cell Parameters. Biosensors (Basel). 2018 Oct 30;8(4):102.
[5] Vachali PP, et al. Surface plasmon resonance (SPR)-based biosensor technology for the quantitative characterization of protein-carotenoid interactions. Arch Biochem Biophys. 2015 Apr 15;572:66-72.