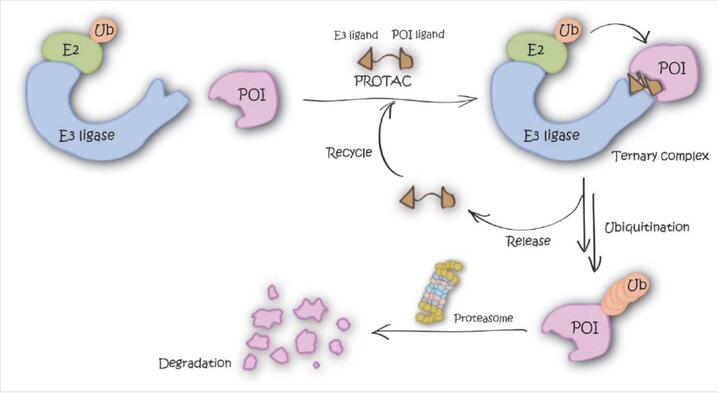
Researchers believe that Targeted Protein Degradation (TPD) technology has emerged as a groundbreaking strategy to revolutionize drug discovery for previously undruggable targets, particularly with the advent of Proteolysis-Targeting Chimeras (PROTACs) based on the ubiquitin-proteasome system, which is one of the representative strategies in this field [1].
However, previous PROTAC technologies have been limited by the availability of E3 ligases and substrate specificity of the proteasome. Through continuous innovation and development by researchers, various novel targeted protein degradation technologies have emerged in recent years. These include Lysosome-Targeting Chimeras (LYTACs), Autophagy-Targeting Chimeras (AUTACs), among others [2].
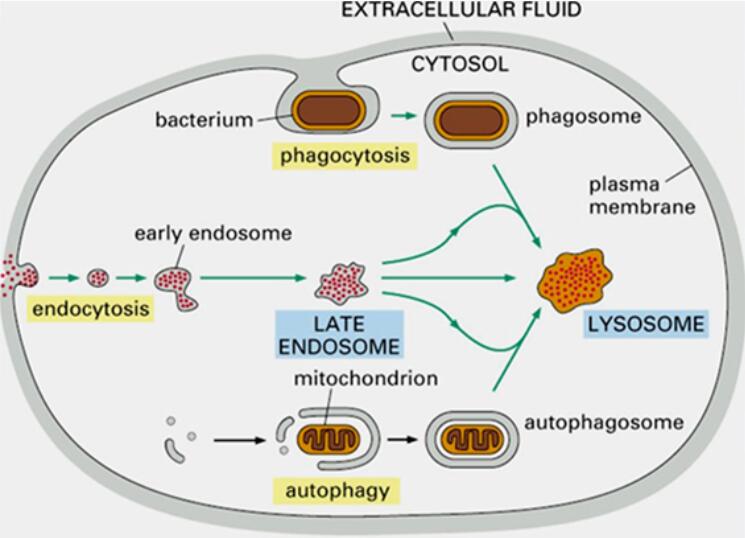
Due to its ability to eliminate disease-causing target proteins through the body's own protein clearance system, Targeted Protein Degradation (TPD) technology offers a broader range of applications compared to traditional small molecule inhibitors [3]. Over the past decade, more than 60 TPD drugs have entered clinical trials, with over a thousand clinical trials initiated. In the past two years, the number of clinical trials for TPD drugs has been increasing rapidly at a rate of 31.5% per year, demonstrating the applicability and attention given to TPD technology.
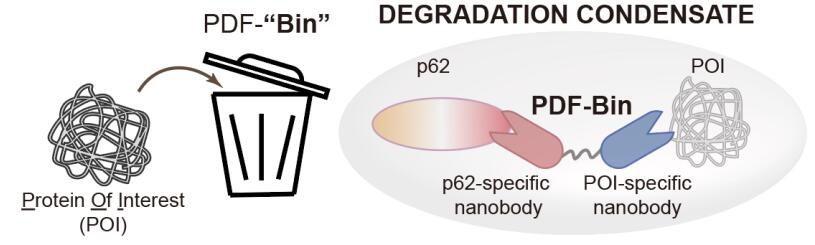
Recently, researchers from Tsinghua University's School of Life Sciences and three marine research institutes have collaborated to achieve the latest advancements in targeted protein degradation technology. Their collaborative research titled "An all-in-one targeted protein degradation platform guided by degradation condensates-bridging bi-specific nanobodies" was published in the journal Cell Research. This research introduces a novel targeted protein degradation technology called PDF-Bin. The innovation lies in the utilization of a bispecific nanobody, capable of simultaneously binding to both p62 protein and the target protein. This enables the specific recruitment of target proteins to p62 degradation condensates, resulting in efficient targeted degradation.
The technology demonstrates degradation effects on various subcellular localizations and different mobility states of target proteins. Additionally, PDF-Bin selectively determines the optimal degradation pathway for different target proteins. This discovery provides new research avenues for the further development and application of targeted protein degradation technology.
A noteworthy aspect of this research is that the targeted protein degradation (TPD) technology is based on bispecific nanobodies as the targeting strategy. Compared to traditional small molecule or macromolecule-based targeting strategies, nanobodies offer higher specificity and stronger binding affinity, thereby significantly reducing off-target risks and providing a solid foundation for future TPD drug development.
Since their discovery, nanobodies have garnered considerable attention from scientists due to their small molecular size, excellent protein stability, high affinity, strong specificity, and potent targeting capabilities, offering extensive research and application prospects.
In 2022, a study published in ACS Cent. Sci. by Professor Laura M.K. Dassama from Stanford University and Professor Stuart H. Orkin from Harvard Medical School demonstrated the use of nanobodies to obtain protein ligands selective for the BCL11A protein. This strategy involved fusing nanobodies to a cell-permeable mini-protein and an E3 adapter to produce a degrader capable of depleting cellular BCL11A in differentiating primary erythroblast cells, thereby inducing fetal hemoglobin expression. This research provided scholars with a reversible and transient method of inducing fetal hemoglobin expression by temporarily regulating BCL11A [4].
These studies collectively showcase the robust application capabilities of nanobodies in the field of TPD drug development. It is anticipated that in the near future, more novel strategies for targeted protein degradation based on nanobodies will emerge, offering further research directions and foundations for TPD drug development.