Currently, nanobody pipelines are widely distributed globally, covering multiple areas including central nervous system diseases, circulatory system diseases, infectious diseases, tumors, and autoimmune diseases.
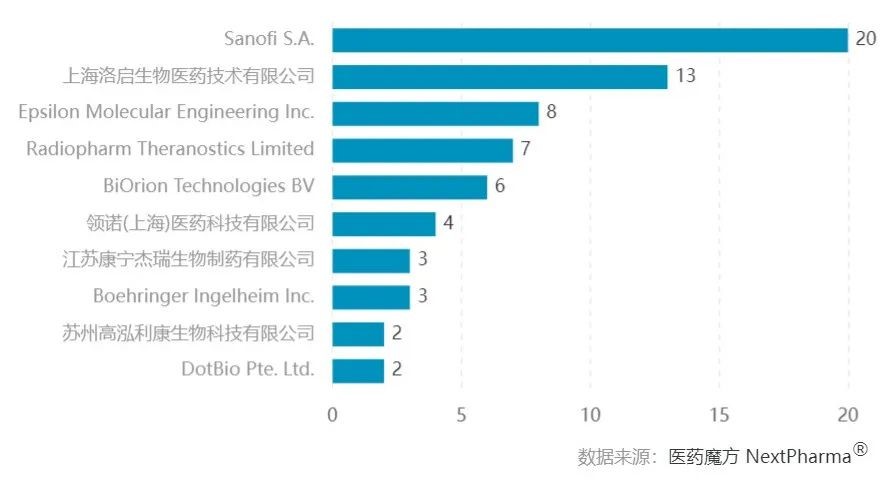
According to data from PharmaCube, as of 2023, there are 238 research pipelines related to nanobodies in the global biopharmaceutical industry, with the majority still in the preclinical stage. Sanofi holds the leading position globally with the acquisition of Ablynx, boasting 20 pipelines, while Shanghai Luqi Bio has the second position with 13 pipelines.
 Nanobodies
Nanobodies
Top 10 Distribution of Global Enterprises Involved in Nanobody Research Pipeline Quantity
According to data from the ClinicalTrial website in the United States, there are currently 20 clinical trials underway for multi-specific nanobody pipelines, primarily focused on oncology. These pipelines are predominantly in Phase I and Phase II clinical trials.
In China, research on nanobodies closely mirrors international trends, with a primary focus on oncology followed by autoimmune diseases. However, there is a notable difference in the development stage of domestic nanobody research compared to the international arena. While Chinese nanobody research is aligned with international standards, the progress in the developmental stages lags behind, with most nanobody drug pipelines in China still in the preclinical phase.
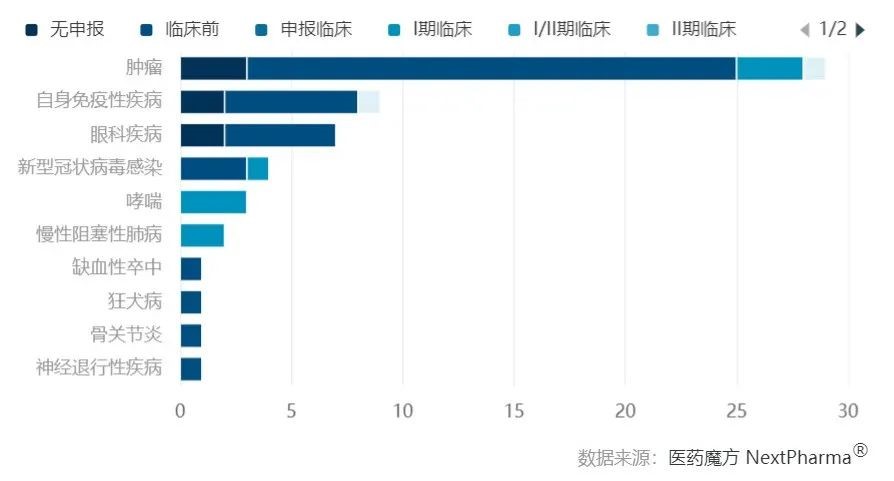
Top 10 Distribution of Indications for Domestic Nanobody Development Pipelines
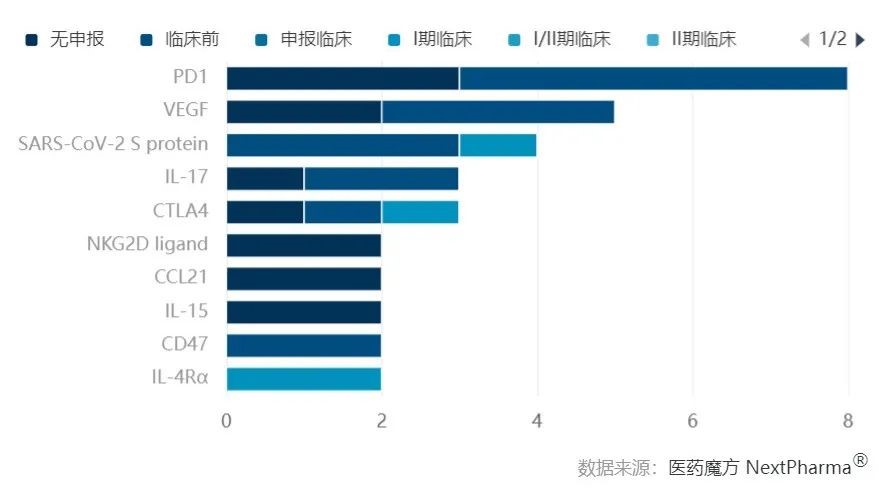
Top 10 Distribution of Targets for Domestic Nanobody Development Pipelines
The domestic landscape of nanobody research and development in China, although not as early as internationally, has gradually gained momentum, accompanied by the continuous efforts of domestic medical scientists to excel. According to incomplete statistics from YaoYao Magic, China's nanobody development pipelines now account for approximately one-fourth of the global total.
In the field of oncology, nanobodies in China have demonstrated significant application value. As previously mentioned by Nanbo Life in earlier articles, domestically developed nanobody drugs have successfully entered the market. KN035, an independently developed nanobody by Koning Jie, was approved for marketing in China, becoming the country's first marketed nanobody drug. With sales revenue exceeding 350 million RMB in H1 2023 alone, Koning Jie recorded revenue of 117 million RMB in the same period. The other two partners of this drug, Smartee and Antsound Pharmaceuticals, also benefited significantly from this achievement

In the field of infectious diseases, nanobodies have also demonstrated significant potential. For instance, research conducted by BiondVax on the SARS-COV-2 virus suggests that nanobody therapy can significantly reduce viral titers in the lungs, and may even lead to the near-complete elimination of the virus from the lungs. Furthermore, nanobodies, leveraging their advantages of small molecular size and high stability, provide promising research data for the treatment of COVID-19. Studies using mouse models have shown that nebulized nanobody drugs can effectively suppress viral infections, confirming the efficacy and clinical potential of nanobodies.
In addition to the aforementioned areas, research on nanobodies is also expanding into other fields. For example, in central nervous system diseases and cardiovascular diseases, nanobodies, due to their unique characteristics such as high stability and specificity, may offer better therapeutic outcomes. Furthermore, nanobodies are being utilized in the design of novel enzyme systems and for the identification of toxic components in mixtures.
Overall, nanobodies have a broad global research pipeline spanning multiple fields. As research progresses, the application areas of nanobodies continue to expand. In the future, with the continuous development and refinement of nanobody technology, we anticipate greater potential for application in various domains. Nanobo Life will continue to serve human health in this field, providing high-quality nanobody development services to enterprises in China and around the world.