In the field of biomedicine, researchers often utilize immobilization strategies to tether antibodies or target proteins onto a solid-phase carrier surface for immunodetection analysis and screening purposes. However, conventional immobilization methods typically include techniques such as covalent binding and adsorption. Yet, the majority of immobilization methods suffer from issues such as random orientation, low immobilization efficiency, and enzyme susceptibility to deactivation [1,2]. These challenges result in increased research costs and workload, particularly for target molecules or substances with low expression levels, exacerbating the difficulty of scientific investigation.

For instance, researchers have observed that in traditional methods of non-directed enzyme immobilization and recovery, enzymes transition from a free state to a fixed state. Furthermore, commonly used carriers for immobilization are typically hydrophobic materials, which can lead to structural folding of enzyme proteins [3]. This scenario often results in alterations in the functional structure of enzymes, causing decreased enzyme activity and reduced enzyme yield and lifespan [4].
On the other hand, directed immobilization methods typically involve coupling small molecule proteins with carrier proteins or biotin to anchor the target onto suitable solid-phase carrier surfaces. This approach preserves the enzyme's natural structure and other advantages. However, the binding strength between the enzyme and the carrier is relatively weak, making it susceptible to factors such as buffer type, pH, and ion strength. This susceptibility makes it challenging to withstand stringent washing and elution conditions, often resulting in enzyme detachment [5]. Therefore, improved immobilization strategies are still required for such proteins requiring directed immobilization.

Recently, research results on the development of polystyrene surface nanobody-directed immobilization technology, led by the Protein Materials Research Group at the Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences, have been published in Analytical Chemistry.
The study utilized a fully synthetic nanobody library to screen and select nanobody B2, which exhibited high affinity for polystyrene. Saturation binding analysis by ELISA yielded a value of 0.7 nM, while isothermal titration calorimetry showed a value of 15.6 nM. Importantly, the binding process was minimally influenced by hydrophobic and electrostatic effects. Molecular modeling studies revealed a critical aromatic hydrogen bond formed between the Trp51 residue within the CDR2 loop of nanobody B2 and polystyrene at a distance of 2.74Å.
To explore the potential of B2 in nanobody-directed immobilization, the study fused B2 with the cancer embryonic antigen nanobody 11C12, which cannot be passively adsorbed onto the polystyrene surface. This fusion protein achieved effective directed immobilization on polystyrene surfaces within 5 minutes directly from the fermentation broth and demonstrated a linear relationship in chemiluminescence enzyme-linked immunosorbent assay [6].
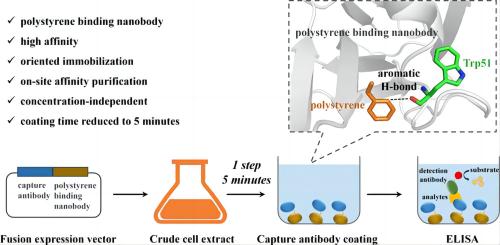
Image: Schematic Representation of the Binding Principle between Trp51 Residue within the CDR2 Loop of Nanobody B2 and Polystyrene Source: ACS Publications
Nanobodies, as a new generation of antibodies, exhibit remarkable characteristics such as high affinity and binding capabilities. These properties have positioned them uniquely as indispensable research tools in fields such as tumor marker detection, diagnostic imaging, and target molecule labeling.
The development of directed immobilization technology based on nanobodies presents a novel and reliable strategy for researchers. This technology ensures the stability of target protein immobilization while preserving protein activity and structural integrity, even for proteins expressed at extremely low levels. It significantly reduces waiting times for target protein screening and detection, enhances yield, expands the scope of protein applications, and ultimately achieves cost-effective research outcomes.
NBLST focuses on the modification and application of nanobodies, striving to establish an integrated experimental public service platform encompassing production, academia, and research. We aim to provide professional and cost-effective experimental services to a wide range of clients, including research institutions, pharmaceutical companies, industrial enterprises, and innovative teams.