According to the World Health Organization's "World Mental Health Report 2022," approximately 1 billion people worldwide suffered from mental health disorders in 2019. In 2020, amidst the outbreak of the COVID-19 pandemic, there was a 25% surge in global cases of depression and anxiety. Over the past decade, mental illnesses have become increasingly prevalent, with conditions such as anxiety disorders, depression, and sleep disorders becoming common occurrences. Research has shown that chronic stress is often associated with these illnesses and can have profound effects on the immune system and the brain's neural pathways, serving as a significant contributing factor to various mental health disorders. Prolonged periods of stress lead to the release of stress hormones such as adrenaline and cortisol, which can affect our nervous system, causing feelings of tension and unease.
Long-term anxiety can lead to psychological and physiological discomfort, affecting our emotions and cognitive abilities. It can result in a loss of interest and motivation in life, giving rise to negative emotions and thoughts. Additionally, it not only impacts personal emotional and mental well-being but also contributes to a range of physical issues, including aggravated sleep disturbances, changes in appetite, and psychological fatigue.
These conditions can significantly disrupt individuals' lives, affecting their social interactions and work performance.
In previous research on patients with severe depression related to chronic stress, scientists have observed an increase in pro-inflammatory cytokines and white blood cells in the circulatory system. Additionally, experiments with mice have shown that stress can compromise the blood-brain barrier, allowing circulating proteins to enter regions like the hypothalamus. Researchers believe that interleukins released by microglial cells control the function and behavior of neurons.
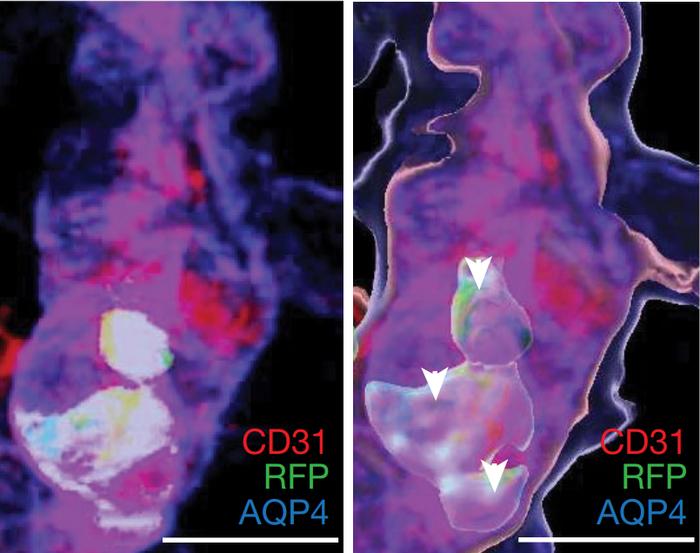
Under stress, monocytes accumulate in cerebral blood vessels. [Reference: [1]]
However, a recent article published in Nature by a research team from the Icahn School of Medicine at Mount Sinai (ISMMS) in the United States proposes a different perspective. They suggest that peripheral immune cells, such as monocytes released from the bone marrow into the bloodstream, upon entering the hypothalamus of the brain, release proteases that alter neuronal function, ultimately leading to behavioral changes characteristic of depression.
In experiments conducted by researchers at ISMMS using a mouse model subjected to social psychological stress, they found that these mice exhibited heightened stress responses and physiological disturbances such as metabolic syndrome and systemic inflammation under conditions of chronic stress. High-dimensional phenotypic analysis of different immune cell subtypes in the circulatory system and brain of the mice revealed a specific increase in pro-inflammatory monocytes but no significant increase in microglial cells, nor did they exhibit pro-inflammatory characteristics.
Furthermore, ISMMS researchers observed a significant increase in the expression of matrix metalloproteinase 8 (MMP8) in both the circulatory system and monocytes entering the brain of stress-sensitive mice. Additionally, gene expression analysis of patients with severe depression revealed that MMP8 was one of the most highly expressed genes.
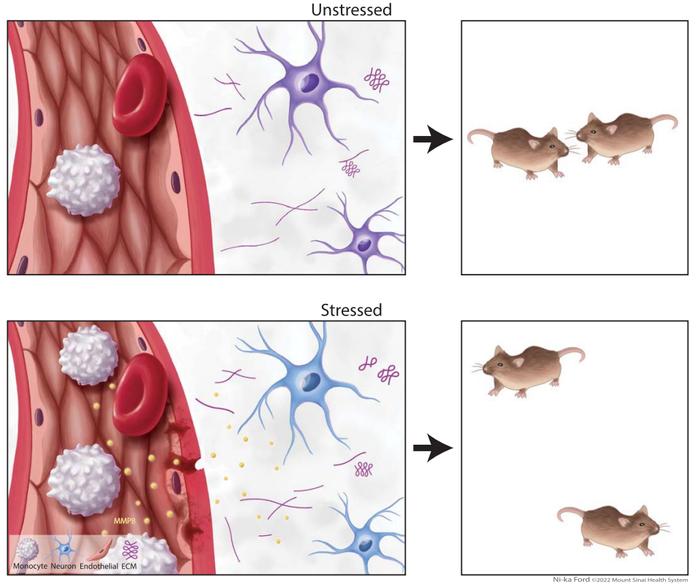
Schematic diagram illustrating the mechanism by which the peripheral immune system influences neuronal function and behavior. Reference: [1]
Research has confirmed that chronic stress leads to a decrease in the expression of endothelial tight junction protein claudin-5 in stress-sensitive mice, resulting in increased blood-brain barrier permeability. Consequently, MMP8 can enter the brain through the compromised blood-brain barrier, directly infiltrating the nucleus accumbens core, altering its neurophysiology, and modulating the ultrastructure of the extracellular space. This ultimately damages social reward behavior, leading to depressive symptoms. Removal of MMP8 in stress-sensitive mice resulted in a reduction in stress-induced social avoidance and no adverse changes in their nucleus accumbens and extracellular space.
This study provides a novel research direction for understanding depression caused by chronic stress. It offers a new strategy for investigating drugs targeting MMP8 secretion from peripheral immune cells, which could potentially lead to innovative therapies for stress-related neuropsychiatric disorders.
Indeed, nanobodies have made significant contributions to research on mental disorders in the past.
Researchers from the Johns Hopkins University School of Medicine and the University of Michigan, Ann Arbor, utilized engineered PFFNB2 nanobodies in their study to target the abnormal folding of alpha-synuclein, forming Lewy bodies.
Due to their extremely small molecular size, nanobodies can easily penetrate the blood-brain barrier and enter neuronal membranes, where they bind to alpha-synuclein and degrade aggregates. Research findings indicate that PFFNB2 nanobodies exhibit remarkable accuracy and stability when binding. After expression in the cerebral cortex, PFFNB2 nanobodies specifically target alpha-synuclein aggregates and can prevent subsequent abnormal folding and spreading of alpha-synuclein.
Indeed, envisioning a research strategy that utilizes nanobodies to target MMP8 for drug development may open up new avenues for treating stress-related disorders.
References:
[1] Cathomas, F., Lin, HY., Chan, K.L. et al. Circulating myeloid-derived MMP8 in stress susceptibility and depression. Nature 626, 1108–1115 (2024).
[2]Mount Sinai study shows that circulating immune cells drawn to the brain during stress can control emotional behaviors. Retrieved Mar. 13, 2024
[3]R. Y B ,Yuqing L ,Ramhari K , et al.α-Synuclein fibril-specific nanobody reduces prion-like α-synuclein spreading in mice[J].Nature Communications,2022,13(1):4060-4060.