The Chinese Journal of Preventive Medicine points out that the incidence of depression in women in China is much higher than that in men, and the incidence rate among individuals aged 15–19 is higher than that in other age groups [1]. Results from the China Mental Health Survey show that the lifetime prevalence of depressive disorders among Chinese adults is 6.8%, including 3.4% for major depressive disorder, corresponding to approximately 95 million patients [2]. Anxiety disorders often co-occur with depression: about 60%–90% of depressed patients have comorbid anxiety disorders, while approximately 50% of anxiety patients experience comorbid depression. The comorbidity rate of the two disorders ranges from 20% to 80%. Both conditions carry a high risk of suicide and disability. According to statistics on depressed patients admitted to the Department of Psychiatry of Lishui Second People’s Hospital, the proportion of depressed patients with suicidal ideation is as high as 54.46% [3], and eventually about 15% of patients die by suicide. This outcome is a profound and amplified destruction and harm to both families and society.

In current medical research, these diseases are generally considered to be highly associated with genetics, brain lesions, acquired living environments, and other diseases, but their specific pathogenesis has not yet been fully clarified. The causes of depression are extremely complex; clinical observations have found that its occurrence and development are related to multiple factors. In addition to the commonly recognized genetic factors, living environment, and psychological influences, its development may also be associated with abnormal lipid metabolism, sleep disorders, cognitive impairment, and so on. Neuroimaging studies have shown that specific brain regions in depressed patients exhibit focal functional and structural abnormalities, including the hippocampus, medial prefrontal cortex (MPFC), dorsolateral prefrontal cortex (DLPFC), anterior cingulate cortex (ACC), posterior cingulate cortex, precuneus (PCC/PCU), amygdala, and caudate nucleus [4]. Studies suggest that the hippocampus and prefrontal cortex are key brain regions involved in the pathogenesis of depression [5]; relevant research also indicates that connection disorders, functional disturbances, or structural changes in multiple brain neural networks—such as structural alterations in the anterior cingulate cortex-thalamus, prefrontal cortex-thalamus, and between prefrontal regions—all demonstrate an association between depression and brain neural networks [6].
At present, drug types for depression mainly include tricyclic/tetracyclic antidepressants, selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), and monoamine oxidase inhibitors (MAOIs). Well-known examples include fluoxetine, fluvoxamine, venlafaxine, maprotiline, and duloxetine. However, these drugs are far from meeting current clinical needs and all have varying degrees of adverse reactions in the nervous, digestive, cardiovascular, and endocrine systems—such as headache, dizziness, nausea, dry mouth, tachycardia or orthostatic hypotension, fatigue, anorexia, and difficulty falling asleep. Some drugs may even exacerbate anxiety or cause immune disorders.
Therefore, there is an urgent need to develop new therapeutic drugs with low side effects and high safety for depression and its comorbidities.
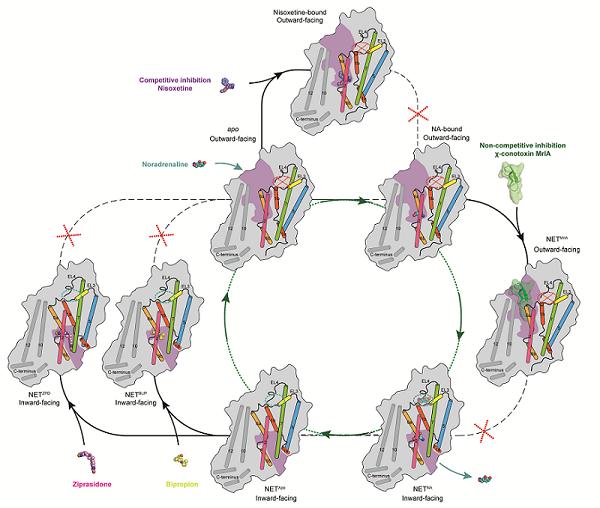
NET Transport Cycle and Multiple Inhibition Modes (Source: Chinese Academy of Sciences)
Norepinephrine (NA) is mainly synthesized and secreted by postganglionic sympathetic neurons and central noradrenergic neurons. As an important neurotransmitter, it plays a crucial role in regulating the active state of the brain and body. When a nerve impulse is transmitted to the nerve terminal, NA is released into the synaptic cleft and binds to receptors on the postsynaptic membrane to exert its physiological effects. The norepinephrine transporter (NET), through active transport, reuptakes unbound NA back into nerve cells for re-storage or metabolism. Dysfunction of this system can lead to diseases such as attention deficit hyperactivity disorder (ADHD), autonomic failure, and depression. For depression, inhibiting NET-mediated reuptake of NA can achieve relatively effective therapeutic effects—examples include bupropion and ziprasidone—but such drugs often cause side effects like nausea, headache, insomnia, and dry mouth.

On July 31, a collaborative study by the team of Zhao Yan from the Institute of Biophysics, Chinese Academy of Sciences (CAS), and the team of Claus Loland from the University of Copenhagen (Denmark) used single-particle cryo-electron microscopy to resolve the high-resolution structures of the human norepinephrine transporter (NET) in different states. This study revealed the transport mechanism of NET and its binding modes with different drug molecules [7].
Beyond the aforementioned research, earlier key studies in this field include: on May 15, a collaborative team consisting of the research groups of Xu Huaqiang, Yang Dehua (Shanghai Institute of Materia Medica, CAS), and Jiang Yi (Lingang Laboratory) deciphered the mechanism of NET homodimerization and transport regulation; on August 14, the team of Wu Jingxiang from the Institute of Materia Medica, Chinese Academy of Medical Sciences, resolved the structure of the human norepinephrine transporter (hNET), further revealing drug design strategies targeting hNET [8].
Each of these studies has its unique strengths, providing a structural basis for the development of new drugs. They are conducive to the development of more effective therapeutic drugs with fewer side effects for diseases such as depression, ADHD, and for pain relief.


On another front, studies have long confirmed that stress can cause dysfunction of the brain’s nervous system and gradually induce chronic low-grade inflammatory responses, thereby leading to depression. Earlier, a research team from the National Institute of Neuroscience (Japan) proposed that chronic stress increases the expression of vascular endothelial growth factor (VEGF) in the blood, causing blood-brain barrier (BBB) dysfunction [9]. More recently, a study by a research team from the Icahn School of Medicine at Mount Sinai revealed that depression arises when monocytes released by the bone marrow into the blood enter brain regions such as the nucleus accumbens; the proteases secreted by these monocytes alter neuronal function, ultimately leading to dysfunction of the "reward system" and the development of depression [10].
It can thus be inferred that abnormal increases in BBB permeability allow substances (e.g., cells) that would otherwise be unable to cross the BBB to easily enter the brain, altering neuronal structure and function—ultimately leading to depression and its related diseases. A major cause of BBB dysfunction is closely associated with VEGF.
VEGF is a highly specific pro-angiogenic factor that enhances vascular permeability, promotes vascular endothelial cell migration, and is also known as vascular permeability factor (VPF). It is involved in the development of various angiogenesis-dependent diseases, such as cancer and diabetic retinopathy. VEGF has multiple subtypes and receptors, among which VEGF-R2 is the most physiologically active receptor, mediating almost all of VEGF’s physiological functions. VEGF itself can induce hippocampal neuron proliferation, promote axon growth, and increase dendritic spine density—processes mainly mediated by VEGF-R2 through signaling pathways such as PI3K/Akt and MAPK/ERK.
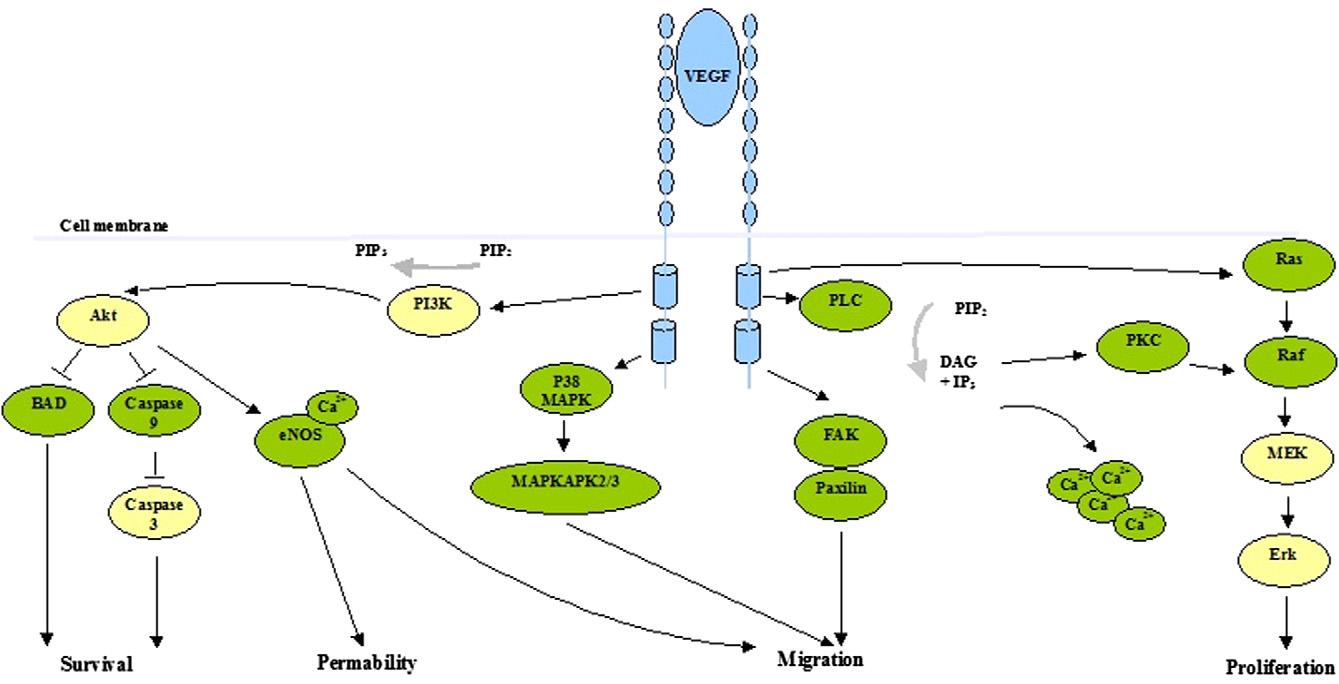
VEGFR2-Mediated Signal Transduction [12]
Scholars, through studies on mice with chronic stress models, found that the expression levels of VEGF in the serum and hippocampus of these mice were significantly higher than those in the control group. Meanwhile, abnormal binding between VEGF and VEGF-R2 erroneously increases BBB permeability, which plays a key role in triggering the series of processes leading to depression. Therefore, VEGF/VEGF-R2 may be a potential target and diagnostic marker for treating depression associated with BBB dysfunction [9].
However, other studies have shown that after receiving antidepressant treatment, the serum VEGF expression level in depressed patients is lower than that in healthy individuals, and the expression level is positively correlated with disease severity and age [11]. Researchers from the Faculty of Pharmacy, Medical University of Silesia (Poland), found in experiments that the use of VEGF-R2 antagonists could completely or significantly block electroconvulsive shock-induced cell proliferation in the subgranular zone (SGZ) of the hippocampal dentate gyrus, and completely abrogated the antidepressant effects of fluoxetine and desipramine [12]. Although this study still confirms the potential research value of VEGF-R2 as a target, its results significantly differ from those of the aforementioned studies.
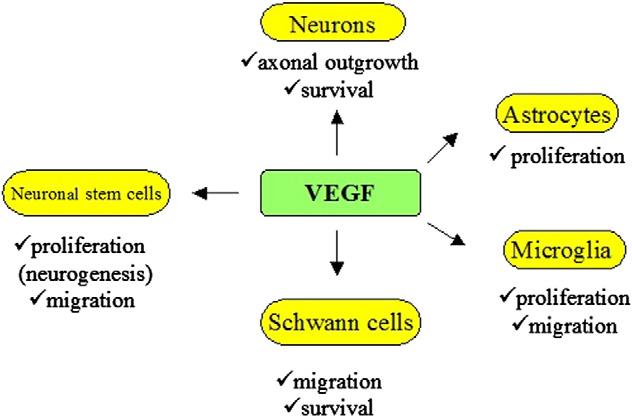
Pleiotropic Effects of VEGF on Central and Peripheral Neurons [12]
Currently, there are multiple research directions for antidepressant development. Among them, studies targeting VEGF/VEGF-R2 in depressive disorders show large discrepancies in processes and results, making it impossible to fully confirm the fundamental relationship between the two. Nevertheless, feasible future research pathways include: using VEGF as a marker for diagnosing depression or evaluating antidepressant efficacy; investigating whether VEGF-R2 inhibition can serve as an adjuvant antidepressant therapy for depression caused by increased BBB permeability; and, after inhibiting BBB permeability, using nanobodies as drug carriers or therapeutic agents themselves to perform reparative treatment on brain regions such as the hippocampus or nucleus accumbens. With advantages such as small size and high specificity, nanobodies show great potential in crossing the BBB, providing new ideas and methods for depression treatment.
In addition, there are studies on the mechanism of GABAergic neuron dysfunction in the prefrontal cortex, as well as research on antidepressant or comorbidity treatment targeting the gut microbiota and astrocytes. Each of these studies makes significant contributions to antidepressant research.
At the same time, we should also focus on populations at high risk of depression, strengthen prevention efforts for depression and its comorbidities, and help depressed individuals break free from their predicament and reintegrate into families and society.

NBLST focuses on the development, modification, and application of nanobodies. It has an alpaca breeding base that meets laboratory animal standards and an independent experimental base. NBLST is committed to building an integrated industry-academia-research public experimental service platform, aiming to provide more professional and cost-effective experimental services for a wide range of biological research institutions, pharmaceutical R&D enterprises, and innovation teams. Researchers from all sectors are welcome to communicate and contact us.
References:
[1]江笑寒,曾智.中国儿童青少年抑郁症疾病负担变化趋势分析[J].中国预防医学杂志,2024,25(03):379-384.DOI:10.16506/j.1009-6639.2024.03.021.
[2] Huang Y, Wang Y, Wang H, et al. Prevalence of mental disorders in China:a cross-sectional epidemiological study [J]. Lancet Psychiatry,2019,6 (3):211-224.
[3]陈静 & 施旭爱.抑郁症患者自杀意念调查及其与家庭功能的关系:情绪调节自我效能感的中介作用.中国健康心理学杂志1-12.
[4] ROLLS E T.Attractor cortical neurodynamics, schizophrenia, and depression[J].Transl Psychiatry,2021,11(1):215.
[5]牟静平,成财,梅兰,等 . 抑郁症灰白质表面积性别差异研 究 [J]. 磁共振成像,2021,12(1):21-26,37.
[6]李芃菲, et al."抑郁症发病的脑功能机制研究进展."中国医学创新 21.05(2024):165-169.
[7]Hu T, Yu Z, Zhao J, Meng Y, Salomon K, Bai Q, Wei Y, Zhang J, Xu S, Dai Q, Yu R, Yang B, Loland CJ, Zhao Y. Transport and inhibition mechanisms of the human noradrenaline transporter. Nature. 2024 Aug;632(8026):930-937. doi: 10.1038/s41586-024-07638-z. Epub 2024 Jul 31. PMID: 39085602.
[8]Ji W, Miao A, Liang K, Liu J, Qi Y, Zhou Y, Duan X, Sun J, Lai L, Wu JX. Substrate binding and inhibition mechanism of norepinephrine transporter. Nature. 2024 Aug 14. doi: 10.1038/s41586-024-07810-5. Epub ahead of print. PMID: 39143211.
[9]Matsuno H, Tsuchimine S, O'Hashi K, Sakai K, Hattori K, Hidese S, Nakajima S, Chiba S, Yoshimura A, Fukuzato N, Kando M, Tatsumi M, Ogawa S, Ichinohe N, Kunugi H, Sohya K. Association between vascular endothelial growth factor-mediated blood-brain barrier dysfunction and stress-induced depression. Mol Psychiatry. 2022 Sep;27(9):3822-3832. doi: 10.1038/s41380-022-01618-3. Epub 2022 May 26. PMID: 35618888.
[10]Cathomas F, Lin HY, Chan KL, Li L, Parise LF, Alvarez J, Durand-de Cuttoli R, Aubry AV, Muhareb S, Desland F, Shimo Y, Ramakrishnan A, Estill M, Ferrer-Pérez C, Parise EM, Wilk CM, Kaster MP, Wang J, Sowa A, Janssen WG, Costi S, Rahman A, Fernandez N, Campbell M, Swirski FK, Nestler EJ, Shen L, Merad M, Murrough JW, Russo SJ. Circulating myeloid-derived MMP8 in stress susceptibility and depression. Nature. 2024 Feb;626(8001):1108-1115. doi: 10.1038/s41586-023-07015-2. Epub 2024 Feb 7. PMID: 38326622; PMCID: PMC10901735.
[11] Rigal, A., Colle, R., Asmar, K.E., et al. (2020) Lower Plasma Vascular Endothelial Growth Factor A in Major Depres- 吉荣图,郑兰兵DOI: 10.12677/acm.2024.1441347 2699 临床医学进展 sive Disorder Not Normalized after Antidepressant Treatment: A Case Control Study. The Australian and New Zealand Journal of Psychiatry, 54, 402-408. https://doi.org/10.1177/0004867419893433
[12]Nowacka MM, Obuchowicz E. Vascular endothelial growth factor (VEGF) and its role in the central nervous system: a new element in the neurotrophic hypothesis of antidepressant drug action. Neuropeptides. 2012 Feb;46(1):1-10. doi: 10.1016/j.npep.2011.05.005. Epub 2011 Jun 29. PMID: 21719103.







