For HSV to successfully infect host cells, it must complete two key steps: "attachment" and "fusion," wherein the gB protein is the core protein executing membrane fusion. It must transition from a "prefusion conformation" to a "postfusion conformation" to complete infection. However, because the prefusion gB protein is a "metastable" structure, it readily and spontaneously converts to the postfusion conformation once removed from the viral membrane. Consequently, scientists have long been unable to resolve its complete structure and have faced challenges in developing targeting molecules that can precisely lock onto this conformation.
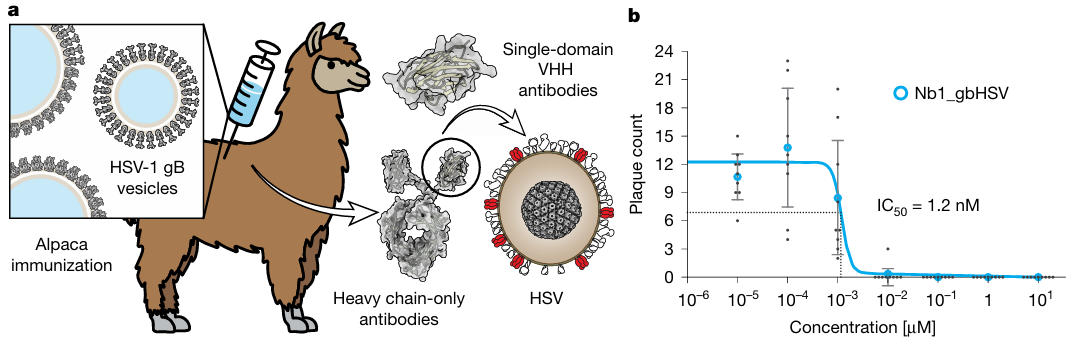
On September 3rd of this year, a collaborative study from institutions including the Centre for Structural Systems Biology (CSSB) in Hamburg and the Leibniz Institute of Virology was published in the journal Nature under the title "A nanobody specific to prefusion glycoprotein B neutralizes HSV-1 and HSV-2". The article reveals that the research team employed an innovative "gB vesicle immunization + phage display" technique to screen and isolate a nanobody, named Nb1_gbHSV, from alpacas that specifically binds the prefusion gB protein. This nanobody not only potently neutralizes HSV-1 with an IC₅₀ of 1.2 nM but also demonstrates cross-serotype binding to HSV-2 gB. Using mutagenesis stabilization and cryo-electron microscopy (cryo-EM) techniques, the team resolved the full-length prefusion structure of HSV-1 gB and the prefusion/postfusion structures of HSV-2 gB for the first time. They revealed that Nb1_gbHSV binds a conformational epitope spanning domains DI, DIII, and DIV, locking gB in its prefusion state to block membrane fusion. This breakthrough provides dual "structural-functional" insights for HSV therapy and vaccine design.
Nanobody Preparation: Simulating the Native Viral State to Overcome Screening Bottlenecks
Traditional antibody preparation often fails due to conformational changes in purified gB. The researchers innovatively adopted a gB vesicle immunization strategy: they transfected BHK-21 cells with the HSV-1 gB gene and collected secreted extracellular vesicles. These vesicles present both prefusion and postfusion conformations of gB on their surface, perfectly mimicking the native state of viral surface proteins. After immunizing alpacas with these vesicles as antigens, they constructed a phage display library and used gB vesicles as "bait" to screen for positive clones.
After two rounds of screening and sequencing validation, 17 candidate nanobodies entered activity testing. In the HSV-1 plaque reduction assay, two nanobodies showed inhibitory activity, but only Nb1_gbHSV remained effective at low concentrations. It inhibited 50% of viral plaque formation at a concentration as low as 1.2 nM (IC₅₀ = 1.2 nM), while the other 16 antibodies were either inactive or required much higher concentrations to take effect. This step not only identified the target nanobody but also demonstrated that the "vesicle immunization" strategy can guide the immune system to recognize the prefusion conformational epitope in its native state.
Figure 1: Nanobody Preparation and Activity Validation
HSV gB Structure Stabilization and Resolution
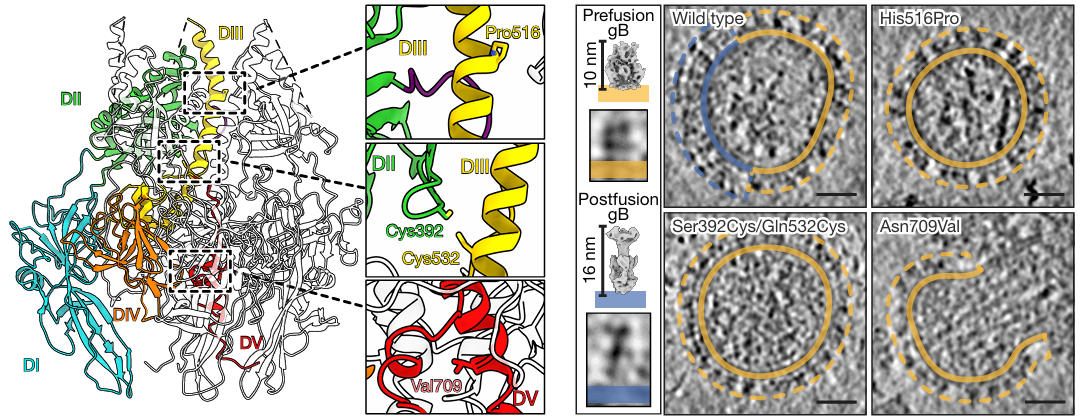
To resolve the structure of prefusion gB, researchers first needed to address its metastability. Based on a previous low-resolution model of HSV-1 gB, they designed three types of stabilizing mutations: 1) Introducing double cysteine mutations (e.g., S392C/Q532C) to form a disulfide bond between DI and DIII, preventing domain separation; 2) Mutating asparagine at position 709 (N709) to valine (V) in Domain V (DV) to enhance helix hydrophobicity and stabilize the trimer core; 3) Retaining the previously discovered H516P mutation, which disrupts the extension capability of the central helix in DIII.
Observation via cryo-electron tomography (cryo-ET) showed that while individual mutations increased the proportion of the prefusion conformation, local conformational instability persisted. Ultimately, the researchers combined the various stabilizing mutations (including the S392C/Q532C disulfide bond, N709V, and H516P) into a single construct, successfully achieving 100% prefusion conformation for the protein on the gB vesicle surface. The results indicated that only the combination of these mutations allowed the protein on the gB vesicle surface to completely maintain the prefusion conformation. These mutations firmly locked the key domains of gB, laying the foundation for subsequent high-resolution structure determination and providing a reusable strategy for stabilizing other metastable viral proteins.
Figure 2: Stabilizing the HSV gB Prefusion Conformation
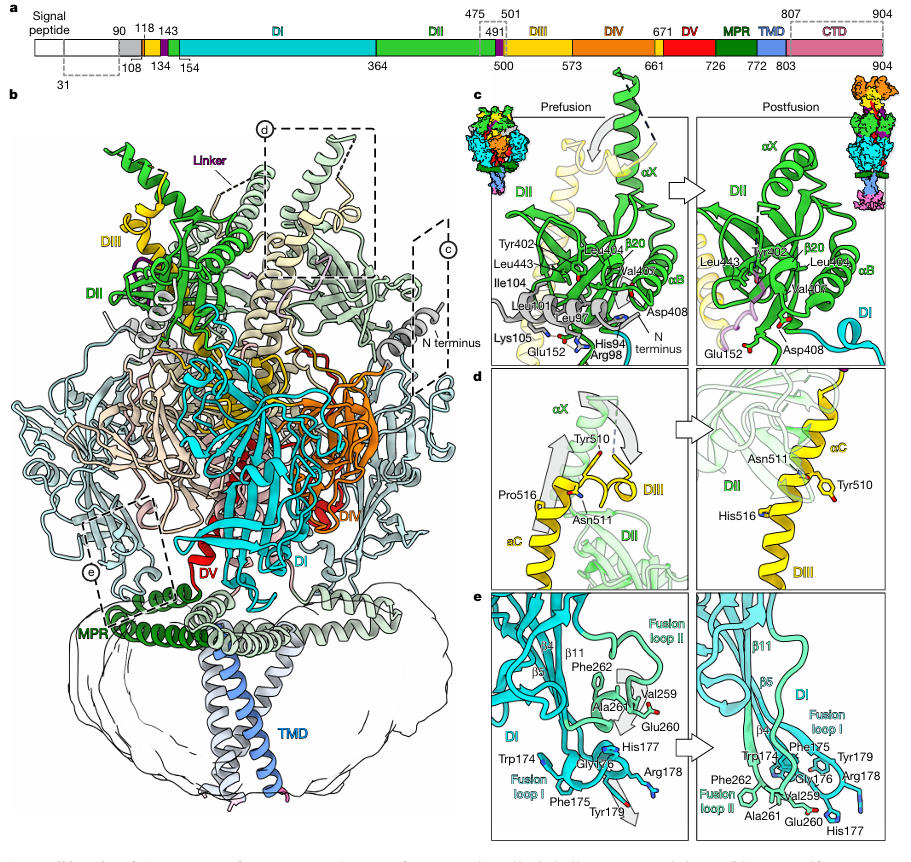
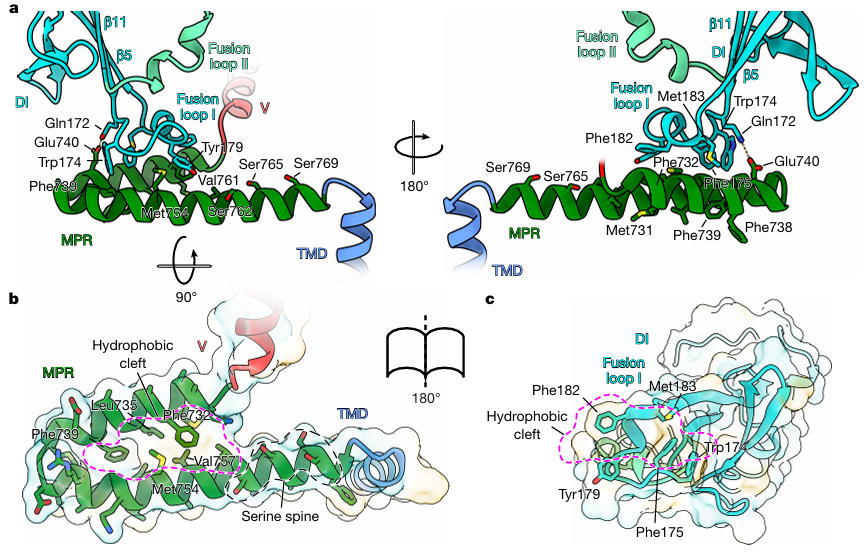
The research team reconstituted the stabilized HSV-1 gB into peptidiscs and then resolved the prefusion structure to a resolution of 2.74 Å using single-particle cryo-EM (Figure 3). This structure revealed several previously unknown key features for the first time: The N-terminal residues 90-106 form an alpha helix that inserts into a groove between DI and DII, stabilized by hydrophobic interactions (L97, I104) and a salt bridge (R98-E152); Fusion loop I (residues 174-179) is not a linear structure as traditionally thought but coils into a 2-turn 3₁₀ helix, precisely embedding into the hydrophobic cleft of the Membrane Proximal Region (MPR) (Figure 4).
Figure 3: Full-Length Prefusion Structure of HSV-1 gB
Figure 4: Interaction between MPR and Fusion Loop
More crucially, when the team incubated Nb1_gbHSV with the stabilized gB and resolved the complex structure to 3 Å resolution (Figure 5), the results showed that Nb1_gbHSV's binding epitope is highly unique. It simultaneously spans three domains: DI, DIII, and DIV. These regions are proximate due to structural compactness in the prefusion conformation but become separated at different locations upon gB rearrangement in the postfusion conformation. Microscale thermophoresis measurements confirmed that Nb1_gbHSV binds prefusion gB with high affinity (K_D ≈ 14 pM), while showing no binding capability to postfusion gB, explaining its mechanism of conformational-specific neutralization.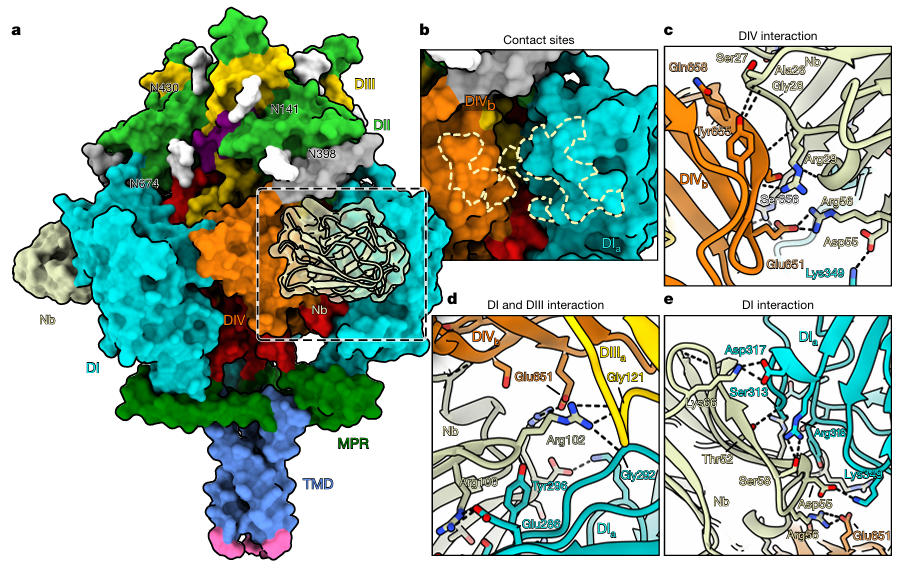
Figure 5: Binding Mode of Nb1_gbHSV with gB
Cross-Serotype Validation: "Broad-Spectrum Protection" from HSV-1 to HSV-2
Considering HSV-2 is the primary pathogen for genital herpes, the research team further validated the effect of Nb1_gbHSV on HSV-2. In cell co-localization experiments, Alexa 647-labeled Nb1_gbHSV co-localized with sfEGFP-tagged HSV-2 gB on the membrane of BHK-21 cells, but showed no binding to gB from VZV (Varicella-Zoster Virus) or HCMV (Human Cytomegalovirus), demonstrating its cross-serotype specificity.
More importantly, the team successfully resolved the structures of both prefusion and postfusion HSV-2 gB. Comparative analysis revealed that the prefusion conformation of HSV-2 gB is highly similar to that of HSV-1, especially the residue epitope bound by Nb1_gbHSV, which is completely conserved. This provides a structural basis for its cross-serotype neutralizing capability. In the cryo-EM density map, density for Nb1_gbHSV was only observed on the prefusion conformation of HSV-2 gB, further confirming its ability to lock HSV-2 gB in the prefusion state.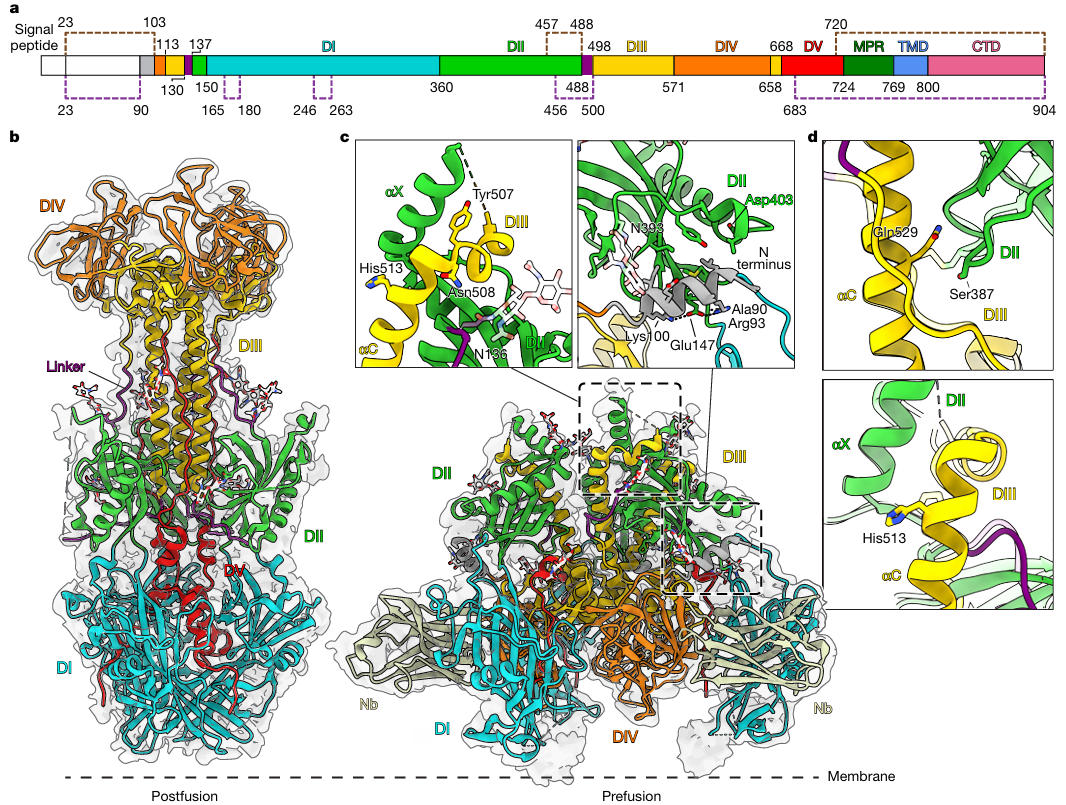
Figure 6: HSV-2 gB Prefusion Structure Bound by Nb1_gbHSV
In the past, the elusive nature and drug resistance of HSV made it difficult for traditional antibodies to achieve significant breakthroughs in this field. Coincidentally, the potential of nanobody technology in HSV treatment has recently been demonstrated by multiple sources. In May of this year, research published in Nature by a team from the University of Science and Technology of China showed that they also developed a bispecific nanobody, Nb14-32-Fc, targeting HSV's gD protein as the core target. Using an innovative design of "dual epitope synergy + Fc segment functional enhancement," this nanobody targets two different non-overlapping epitopes on the gD protein. Their synergistic action increased the binding affinity of the bispecific antibody for HSV-2 gD to 24.1 pM, twice as high as singly targeted nanobodies. Crucially, this dual-target mechanism not only potently neutralizes HSV-1/2 both before and after viral attachment but also significantly inhibits cell-to-cell spread of the virus by over 90%. In vivo models showed that a dose of 1 mg/kg resulted in an 80% survival rate in mice with intracranial HSV-1 infection and rapidly healed corneal ulcers caused by acyclovir-resistant strains. Simultaneously, it activates immune effects via the Fc segment, offering potential for intervening in the viral latent microenvironment.
Today, this study from the Centre for Structural Systems Biology in Hamburg and other institutions not only fills a gap in the understanding of the HSV membrane fusion mechanism but also provides a new "structure-function" dual-driven pathway for antiviral therapy and vaccine development. This research further validates the advantages of nanobodies in the anti-HSV field. Nanobodies, leveraging their "small size + high specificity," can precisely target metastable epitopes of HSV. Their characteristics of "cross-serotype activity + low likelihood of resistance" break through the limitations of existing therapeutic antibody research, providing a critical tool for clinical treatment studies.
Combining insights from these two studies, nanobodies are poised to change the current treatment landscape. The technical and structural strategies employed in the research itself will continue to propel the application of nanobodies in a broader range of antiviral fields, contributing to global public health security.

Wuhan Nano Body Life Science and Technology Co. Ltd. (NBLST) is a nanobody industry platform established under the initiative of the Wuhan Industrial Innovation and Development Research Institute. Its headquarters is located in the main building of the Wuhan Industrial Innovation and Development Research Institute in the East Lake High-tech Development Zone, Wuhan. It boasts a 1400 m² independent laboratory in the Precision Medicine Industrial Base of Wuhan Biolake. Additionally, NBLST has established alpaca experimental and transfer bases in Zuoling, Wuhan, and Tuanfeng, Huanggang, both compliant with laboratory animal standards. These bases currently house over 600 alpacas, providing "zero-immunization-background" guaranteed alpaca immunization services for research institutions and antibody drug development companies.
NBLST focuses on the development, engineering, and application of nanobodies, and is dedicated to building an integrated public experimental service platform for production, education, and research. It possesses a full-chain technology platform encompassing antigen preparation (peptides, proteins, and RNA), antibody discovery and engineering, through to biological function validation/screening. The RNA antigens include RNA structurally and sequentially optimized for alpacas. Antibody discovery and engineering services employ multiple technological routes, including phage display, RNA, and mammalian cell display. Through cross-complementation of multiple platforms, it provides flexible antibody discovery and engineering services for pharmaceutical companies and research institutes, facilitating the development of drug reagents.
In addition to its natural nanobody library, NBLST also offers an off-the-shelf immunized library to help clients quickly screen for antibody molecules that meet their needs.
If you require our services, please feel free to contact us via email: marketingdept@nanobodylife.com