Both homodimers (IL-17A/A, IL-17F/F) and the heterodimer (IL-17A/F) can initiate and sustain inflammatory responses. Most traditional monoclonal antibodies target only IL-17A, failing to effectively block the IL-17F-mediated inflammatory pathway, which contributes to suboptimal responses in some patients. Sonelokimab (SLK), a nanobody drug developed by the Swiss biopharmaceutical company MoonLake Immunotherapeutics, is a novel trivalent nanobody. Its three VHH domains enable high-affinity binding to IL-17A, IL-17F, and human serum albumin, respectively. This design allows SLK to simultaneously block signaling mediated by all three dimeric forms (A/A, A/F, F/F) of IL-17A and IL-17F. Clinical data demonstrate that the PASI 90 (almost complete skin clearance) response rate induced by SLK is significantly higher than that of traditional single-target agents. Particularly in patients with moderate-to-severe psoriasis (baseline PASI ≥10), the PASI 100 (complete skin clearance) response rate reached 57.1% at Week 24 in the SLK 120 mg with induction (WI) group, strongly confirming the superior advantage of its dual-target inhibition.
Compared to traditional monoclonal antibodies (~150 kDa), Sonelokimab has a molecular weight of only about 40 kDa, less than one-third of the former. Its small size allows it to penetrate vascular walls and tissue barriers more easily, reaching inflammatory sites in poorly vascularized areas like the synovium and entheses. Concurrently, its third VHH domain specifically binds to human serum albumin. Due to increased local vascular permeability in inflamed tissues, albumin extravasates and accumulates abundantly. This domain effectively guides Sonelokimab to target and accumulate at inflammatory sites, thereby further enhancing local drug concentration and prolonging its half-life. Preclinical studies (e.g., mouse collagen-induced arthritis models) have confirmed that albumin-binding nanobodies accumulate significantly more in inflamed joints compared to non-albumin-binding nanobodies and traditional mAbs, providing solid mechanistic support for the drug's enhanced efficacy.
Recently, results from a global, randomized, double-blind, placebo-controlled Phase II clinical trial (the ARGO study), funded by MoonLake and conducted in collaboration with numerous institutions including the University of Glasgow and the University of Washington, were announced, confirming the significant efficacy and favorable safety profile of Sonelokimab in PsA treatment.
Trial Design and Patient Baseline Characteristics
The study was conducted at 42 clinical centers across eight countries, including Bulgaria, Germany, and the USA. Between December 2022 and May 2023, 265 patients were screened, and ultimately 207 eligible patients with active psoriatic arthritis were randomized into five treatment groups: Sonelokimab 120 mg every 4 weeks (with induction, WI), Sonelokimab 60 mg every 4 weeks (with induction, WI), Sonelokimab 60 mg every 4 weeks (no induction, NI), Placebo (PBO), and an adalimumab active reference arm (ADA, 40 mg every 2 weeks).
Key eligibility criteria included age ≥18 years, presence of active psoriasis or a confirmed history of psoriasis, concurrent diagnosis of PsA according to classification criteria, and active disease defined by ≥3 tender joints (TJC68) and ≥3 swollen joints (SJC66). Exclusion criteria included prior exposure to more than two biologics of any type, and previous treatment failure with IL-17 or TNF inhibitors. At baseline, patient characteristics were well-balanced across treatment groups: 49.3% were female, 17.4% had prior biologic exposure, and 71.0% were receiving concomitant methotrexate (MTX). Disease severity measures related to joints and skin were comparable across groups, establishing a fair foundation for subsequent efficacy comparisons.
Dual Improvement in Joint and Skin Symptoms
Regarding joint symptom improvement, at Week 12, the ACR50 response rates for the Sonelokimab 60 mg WI and 120 mg WI groups were 46.3% and 46.5%, respectively, significantly higher than the placebo group's 20.0% (P < 0.05, P < 0.01). The 60 mg NI group achieved a 36.6% response, which was not statistically significant versus placebo (P = 0.086). By Week 24, the ACR50 response rates in the Sonelokimab dose groups further improved to 58.1%–61.0%. Notably, patients who switched from placebo to SLK 120 mg NI at Week 12 achieved an ACR50 response rate of 54.1% at Week 24, compared to 54.8% in the adalimumab group, fully demonstrating the sustained control of joint inflammation by Sonelokimab.
Regarding skin symptom improvement, at Week 12, the PASI 90 response rates for the Sonelokimab 60 mg WI and 120 mg WI groups were 76.9% and 59.3%, respectively, both significantly superior to the placebo group's 15.4% (P < 0.001, P = 0.003). By Week 24, the PASI 100 response rate reached up to 63.0% in the SLK groups. Particularly noteworthy, patients who switched from placebo to SLK treatment saw their PASI 100 response rate surge from 15.4% at Week 12 to 75.0% at Week 24, demonstrating the powerful skin clearance capability of SLK.
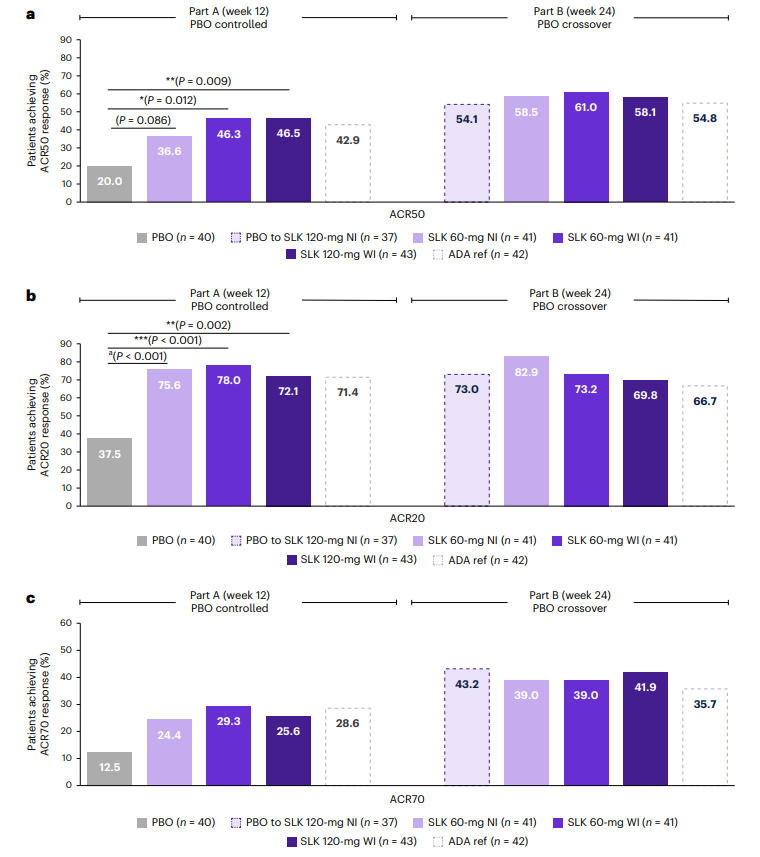
Figure 1: ACR20/50/70 Response Rates Across Treatment Groups at Weeks 12 and 24
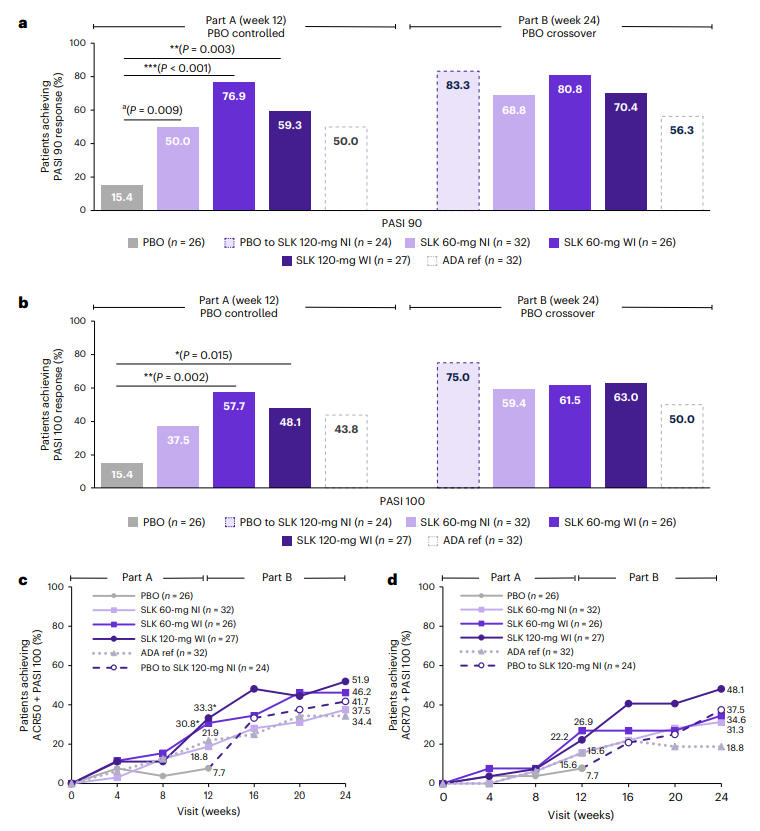
Figure 2: PASI 90/100 Response Rates Across Treatment Groups at Weeks 12 and 24
Multi-Domain Efficacy and Safety
Regarding composite endpoints, at Week 24, the proportion of patients simultaneously achieving ACR70 and PASI 100 was as high as 48.1% in the Sonelokimab 120 mg WI group. Furthermore, the proportion achieving minimal disease activity (MDA) reached 61.0% in the SLK 60 mg WI group, significantly superior to placebo (20.0% at Week 12). Patient-reported outcomes (e.g., PsAID-12, pain scores, Health Assessment Questionnaire Disability Index - HAQ-DI) also showed significant improvements. Efficacy was consistent across key subgroups, including females, overweight patients, and those using concomitant methotrexate, highlighting its broad applicability.
Regarding safety, Sonelokimab was well-tolerated. The most frequent treatment-emergent adverse events were nasopharyngitis (6.1% in the 60 mg group, 5.2% in the 120 mg group) and upper respiratory tract infection (6.1% in the 60 mg group, 4.1% in the 120 mg group). Only four cases (2.1%-2.4%) of mild-to-moderate oral candidiasis were reported. Notably, no events of inflammatory bowel disease (IBD), major adverse cardiovascular events (MACE), or other adverse events of special interest traditionally associated with IL-17 inhibitors were observed, indicating a favorable safety profile.
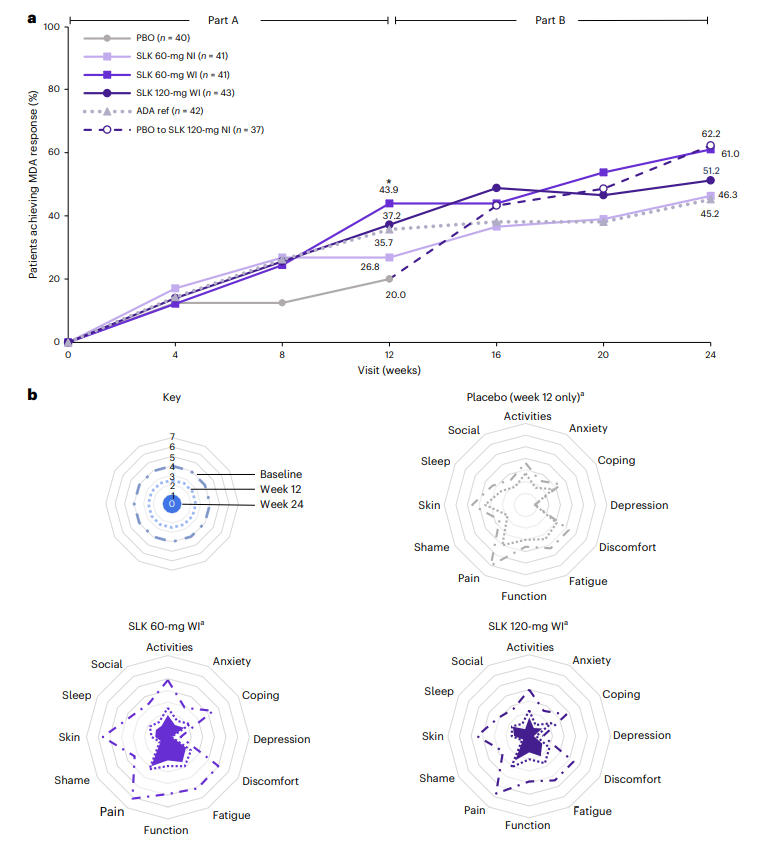
Figure 3: Proportion of Patients Achieving MDA and Patient-Reported Outcomes at Week 24 Across Treatment Groups
Advancing Nanobody Research for Multiple Autoimmune Diseases
The successful Phase II trial of Sonelokimab not only confirms its exceptional efficacy and safety in PsA treatment but also highlights the immense potential of the nanobody technology platform for treating autoimmune diseases. Its unique dual-target inhibition, superior tissue penetration, and modular design offer new avenues for future drug development.
Beyond psoriatic arthritis, the therapeutic strategy of targeting IL-17A/F, exemplified by Sonelokimab, can be rapidly extended to other IL-17 pathway-mediated autoimmune conditions. Research targeting IL-17A and IL-17F is already underway in diseases such as ankylosing spondylitis, rheumatoid arthritis, and atopic dermatitis, with promising interim results. For instance, in ankylosing spondylitis, approximately 40% of PsA patients have axial involvement, and Sonelokimab has already demonstrated a trend towards improvement in axial symptoms (assessed via BASDAI) in PsA patients, providing a preliminary rationale for its investigation in classic ankylosing spondylitis. Furthermore, this strategy offers unique advantages for patients presenting with multiple IL-17-related comorbid conditions, potentially enabling simplified "one-drug-multiple-diseases" treatment regimens, thereby greatly enhancing patient adherence and quality of life.

Wuhan Nano Body Life Science and Technology Co. Ltd. (NBLST) is a nanobody industry platform established under the initiative of the Wuhan Industrial Innovation and Development Research Institute. Its headquarters is located in the main building of the Wuhan Industrial Innovation and Development Research Institute in the East Lake High-tech Development Zone, Wuhan. It boasts a 1400 m² independent laboratory in the Precision Medicine Industrial Base of Wuhan Biolake. Additionally, NBLST has established alpaca experimental and transfer bases in Zuoling, Wuhan, and Tuanfeng, Huanggang, both compliant with laboratory animal standards. These bases currently house over 600 alpacas, providing "zero-immunization-background" guaranteed alpaca immunization services for research institutions and antibody drug development companies.
NBLST focuses on the development, engineering, and application of nanobodies, and is dedicated to building an integrated public experimental service platform for production, education, and research. It possesses a full-chain technology platform encompassing antigen preparation (peptides, proteins, and RNA), antibody discovery and engineering, through to biological function validation/screening. The RNA antigens include RNA structurally and sequentially optimized for alpacas. Antibody discovery and engineering services employ multiple technological routes, including phage display, RNA, and mammalian cell display. Through cross-complementation of multiple platforms, it provides flexible antibody discovery and engineering services for pharmaceutical companies and research institutes, facilitating the development of drug reagents.
In addition to its natural nanobody library, NBLST also offers an off-the-shelf immunized library to help clients quickly screen for antibody molecules that meet their needs.
If you require our services, please feel free to contact us via email: marketingdept@nanobodylife.com