In recent years, advances in large-scale deep sequencing and structural biology technologies, particularly breakthroughs in cryo-electron microscopy (cryo-EM), have provided powerful momentum for structure-based drug discovery. Leucine-rich repeat-containing G protein-coupled receptor 4 (LGR4) has been genetically validated as a potential anti-obesity target due to its crucial role in energy metabolism and adipose tissue browning. However, developing traditional small-molecule antagonists has proven extremely challenging, largely due to the surface characteristics of its extracellular domain (ECD).
Against this backdrop, a collaborative study led by Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine and the Shanghai Institute of Materia Medica, Chinese Academy of Sciences, successfully determined the three-dimensional structures of LGR4 and its complex with ligands. Furthermore, they developed a nanobody, NB21, capable of specifically blocking the LGR4 signaling pathway, offering a novel solution for obesity treatment. These findings have been published in the esteemed international journal Nature Communications.
This research successfully resolved the cryo-EM structures of full-length LGR4 and its complex with RSPO2(FU). The inhibitory nanobody NB21, which blocks the binding of RSPO1/2 to LGR4, was screened and identified, and the structure of the LGR4-NB21 complex was determined. NB21 fused with the mouse IgG2 Fc fragment (NB21-mFc) significantly inhibited the Wnt/β-catenin signaling pathway both in vitro and in vivo, enhanced thermogenesis and mitochondrial respiration in beige adipocytes, promoted the browning of white adipose tissue in vivo, and substantially increased energy expenditure. It demonstrated significant weight-reduction effects in both high-fat diet-induced and genetic (ob/ob) obesity models, providing a novel targeted drug candidate and a structural basis for obesity treatment.
Cryo-EM Structure Determination of LGR4 and Related Complexes
To overcome the technical challenges in determining the LGR4 structure, the research team designed a high-affinity nanobody (referred to as NB52 in the context of structural studies, distinct from the therapeutic candidate NB21). This was fused with the extracellular adhesion domain of Helicobacter pylori HopQ protein to create a 'Megabody' MB52, which served as an auxiliary tool for structural studies, primarily used to increase the particle size of LGR4 complexes and optimize their orientation for cryo-EM analysis. With the assistance of MB52, the structure of full-length LGR4 was determined at a resolution of 3.03 Å. The LGR4 ECD forms a 180-degree extended, curved horseshoe shape, adopting an upright conformation relative to the membrane layer, while its 7-transmembrane domain resembles the inactive conformation of Class A GPCRs.
To investigate the conformational changes induced by ligand binding, the team further resolved the cryo-EM structure of the LGR4-RSPO2(FU) complex at a resolution of 3.06 Å. The results showed that LGR4 and RSPO2(FU) form a 1:1 stoichiometric complex. RSPO2 binds to the concave surface near the top of the LGR4 ECD primarily via its FU2 domain, interacting mainly with LRR3-LRR7 repeats. A stable interaction interface is formed through salt bridges, hydrogen bonds, and hydrophobic interactions, burying approximately 2200.96 Ų of solvent-accessible surface area. Comparative analysis revealed that RSPO2 binding induces only a slight 2.5-degree rotation of the LGR4 ECD and does not trigger a transition to an active conformation.
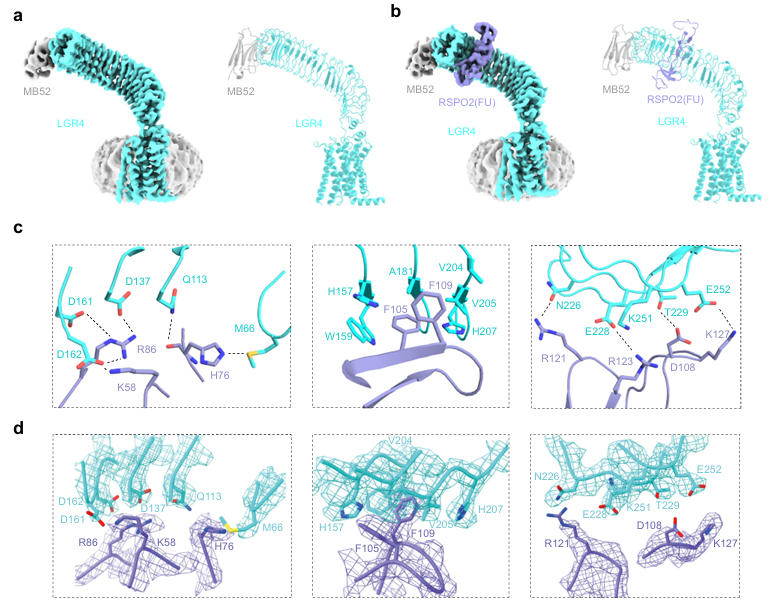
Fig. 1丨Cryo-EM structures of LGR4 alone and in complex with RSPO2(FU).
Screening and Structural Characterization of the Inhibitory Nanobody NB21
Based on a phage display library of LGR4-targeting nanobodies, the research team screened for the nanobody NB21, which exhibited significant inhibitory activity in TOPFlash assays. BLI experiments confirmed that NB21 binds to the human LGR4 ECD with a dissociation constant (K_D) of 1.12 ± 0.04 nM and to the mouse LGR4 ECD with a K_D of 0.82 ± 0.01 nM, demonstrating high affinity and species cross-reactivity. NB21 effectively competed with RSPO1/2 for the binding site on LGR4.
Subsequently, the team determined the cryo-EM structure of the LGR4-NB21 complex (resolution 3.64 Å). They found that NB21 binds to the upper concave surface of the LGR4 ECD through its complementarity-determining regions (CDRs), burying approximately 2260.04 Ų of solvent-accessible surface area. The binding epitope of NB21 highly overlaps with the binding sites of RSPO1/2 on LGR4, providing the structural basis for NB21's ability to block ligand binding. Further analysis revealed that K31 of NB21 forms salt bridges with D137 and D161 of LGR4, Y32 forms a hydrogen bond with D162 of LGR4, and R100 forms a salt bridge with E228 of LGR4. These key interacting residues are fully conserved between human and mouse LGR4, ensuring functional stability across species.
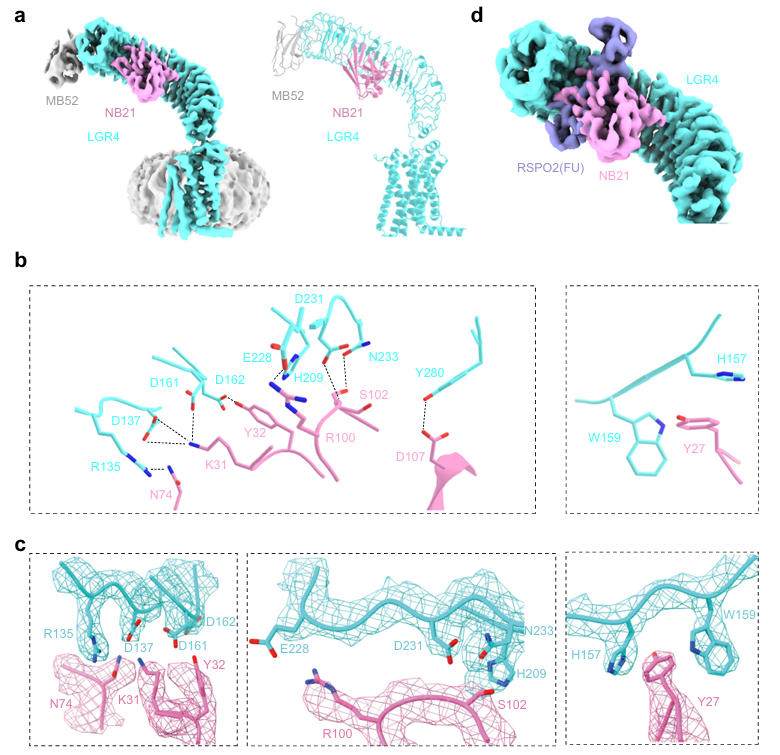
Fig.2丨Cryo-EMstructureandanalysisofLGR4-NB21complex.
In Vitro Functional Validation of NB21-mFc
To improve the in vivo half-life and bioavailability of NB21, the research team constructed the NB21-mFc fusion protein. In vitro experiments using stromal vascular fractions (SVFs) from mouse subcutaneous white adipose tissue (sWAT) showed that treatment with RSPO1 increased active β-catenin levels, promoted its nuclear accumulation, and activated the transcription of target genes in the canonical Wnt signaling pathway. The addition of NB21-mFc significantly inhibited these effects.
In beige adipocytes, NB21-mFc dose-dependently suppressed the activation of the canonical Wnt signaling pathway induced by RSPO1/2, while concurrently upregulating the expression of thermogenic genes and enhancing mitochondrial respiratory function. This indicates that NB21-mFc effectively reverses the inhibitory effect of RSPO1/2 on beige fat thermogenesis and promotes the conversion of white fat to a brown fat-like phenotype.
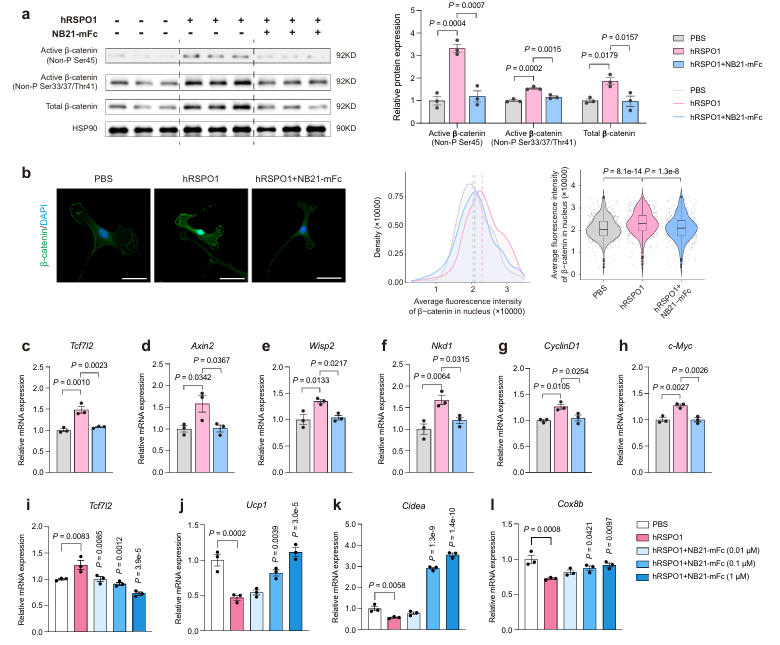
Fig.3 丨 NB21-mFcsuppressescanonicalWnt/β-cateninsignalingpathwayand promotesawhite-to-brownadipocyteswitchinvitro.
In Vivo Validation of the Anti-Obesity Effects of NB21-mFc
To confirm the anti-obesity effects of NB21-mFc in vivo, the research team administered NB21-mFc via intraperitoneal injection (0.2 mg/kg, every other day for 6 weeks) to a high-fat diet (HFD)-induced obesity mouse model. Observations revealed that NB21-mFc significantly reduced body weight gain and fat mass in wild-type (WT) mice, particularly decreasing the weight of visceral white adipose tissue (vWAT) and subcutaneous white adipose tissue (sWAT), reducing adipocyte size, and increasing UCP1 protein expression. Metabolic monitoring showed a significant increase in energy expenditure (EE) in the NB21-mFc treated group, along with enhanced thermogenic capacity under both acute and chronic cold exposure.
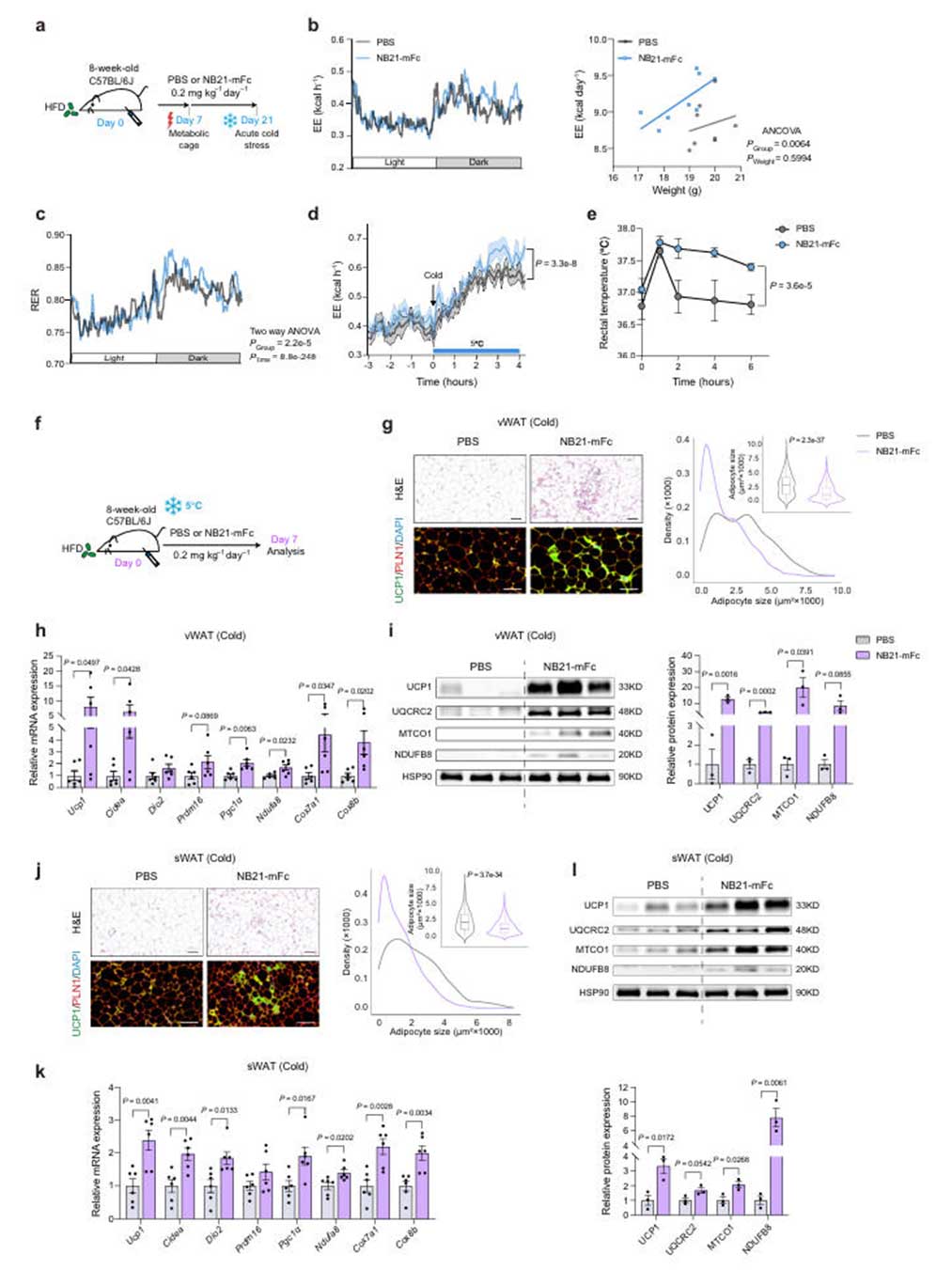
Fig.4 丨NB21-mFcenhancesenergyexpenditureandthermogenesisundercold stress.
In Lgr4 gene knockout (Lgr4<sup>m/m</sup>) mice, the effects of NB21-mFc on promoting thermogenesis and reducing obesity were completely abolished, indicating that its mechanism of action is highly dependent on LGR4. Although NB21 might exhibit cross-reactivity with other LGR family members (such as LGR5/6), gene knockdown experiments confirmed that the metabolic benefits of NB21-mFc are primarily mediated by LGR4.
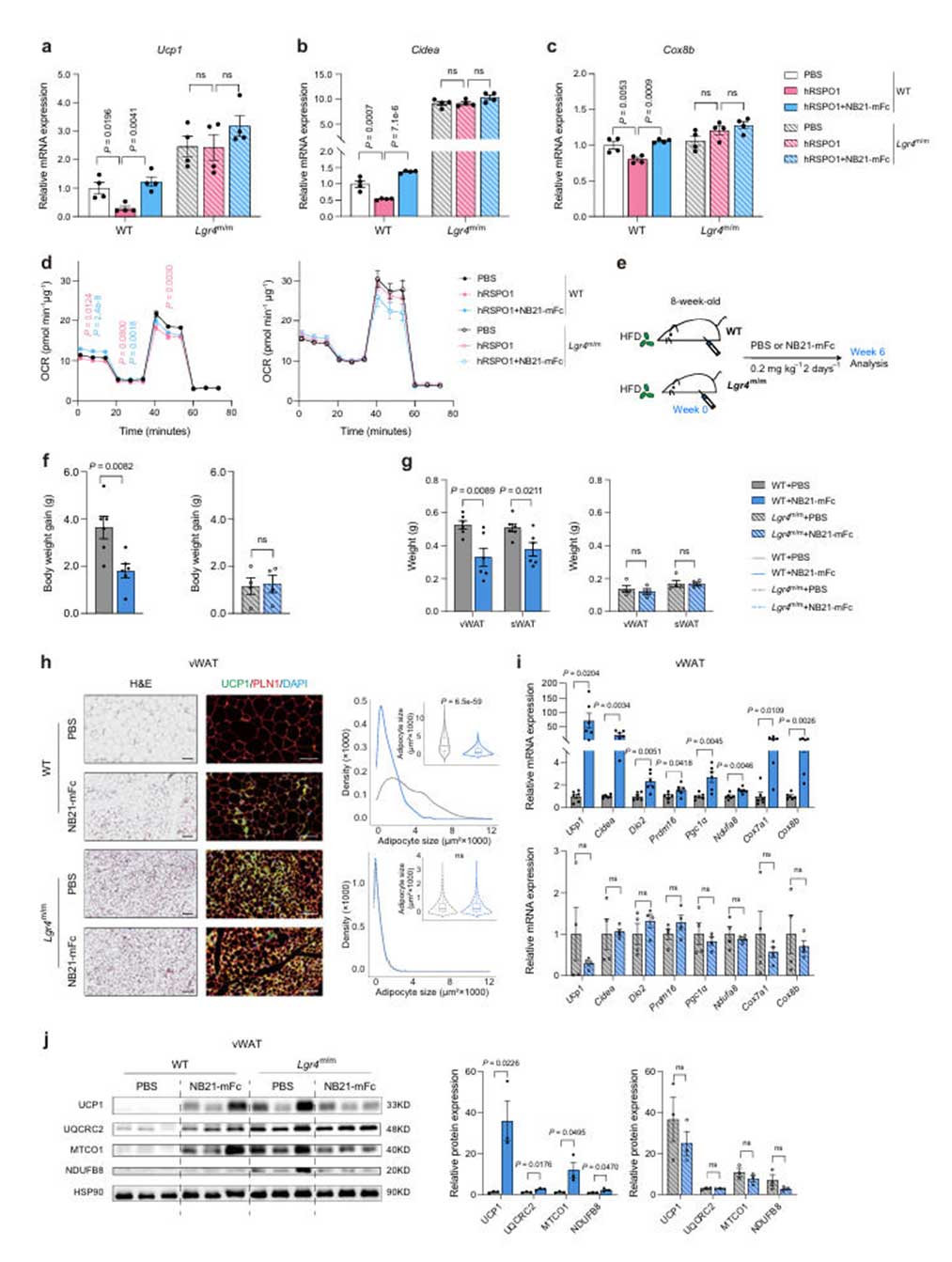
Fig.5 丨NB21-mFc promotes a browning program in an LGR4-dependent man ner.
In a leptin-deficient (ob/ob) obesity mouse model, treatment with low doses of NB21-mFc significantly reduced body weight and fat mass while increasing energy expenditure. Histological and molecular analyses showed increased UCP1 expression and decreased levels of inflammatory factors in the adipose tissue of these mice, further supporting its therapeutic potential in severe genetic obesity models.
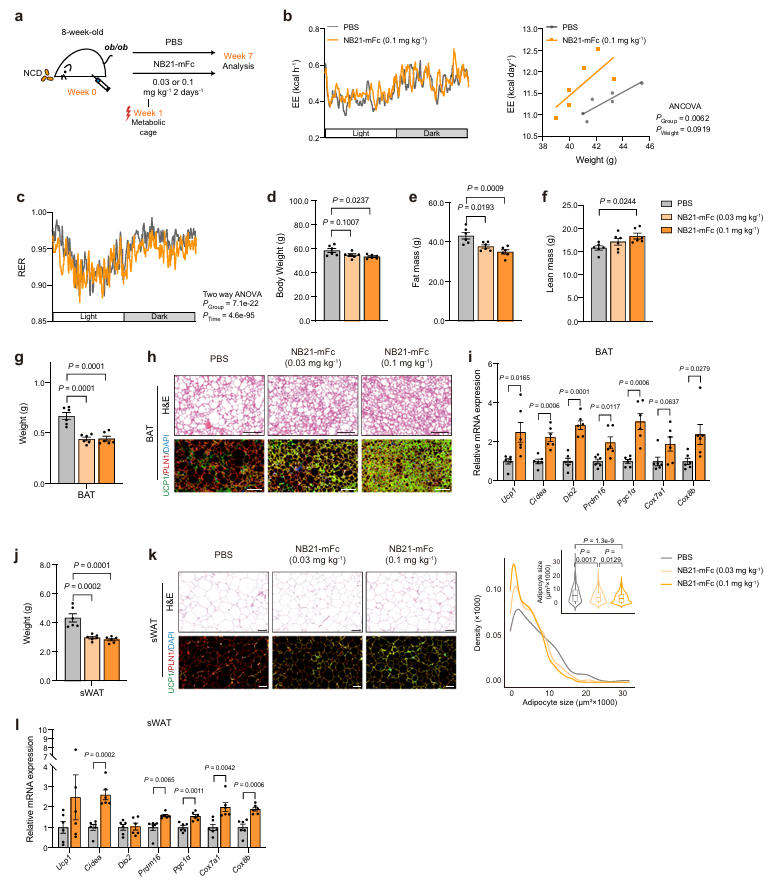
Fig.6 丨Anti-obesityeffectsofNB21-mFcinob/obmice.
This study not only revealed the detailed structures of LGR4 and its ligand complexes, adding a new target to the field of obesity treatment, but also successfully developed the therapeutically promising nanobody NB21. NB21 demonstrated significant weight-loss efficacy in multiple obesity models and holds promise as a next-generation candidate for anti-obesity drugs, offering new hope for obese patients worldwide. Furthermore, this research, led by a Chinese scientific team, deciphered the core structure of a key target and developed a proprietary drug candidate, thereby enhancing international competitiveness in this field and injecting new momentum into the innovative development of China's biopharmaceutical industry.

Wuhan Nano Body Life Science and Technology Co. Ltd. (NBLST) is a nanobody industry platform established under the initiative of the Wuhan Industrial Innovation and Development Research Institute. Its headquarters is located in the main building of the Wuhan Industrial Innovation and Development Research Institute in the East Lake High-tech Development Zone, Wuhan. It boasts a 1400 m² independent laboratory in the Precision Medicine Industrial Base of Wuhan Biolake. Additionally, NBLST has established alpaca experimental and transfer bases in Zuoling, Wuhan, and Tuanfeng, Huanggang, both compliant with laboratory animal standards. These bases currently house over 600 alpacas, providing "zero-immunization-background" guaranteed alpaca immunization services for research institutions and antibody drug development companies.
NBLST focuses on the development, engineering, and application of nanobodies, and is dedicated to building an integrated public experimental service platform for production, education, and research. It possesses a full-chain technology platform encompassing antigen preparation (peptides, proteins, and RNA), antibody discovery and engineering, through to biological function validation/screening. The RNA antigens include RNA structurally and sequentially optimized for alpacas. Antibody discovery and engineering services employ multiple technological routes, including phage display, RNA, and mammalian cell display. Through cross-complementation of multiple platforms, it provides flexible antibody discovery and engineering services for pharmaceutical companies and research institutes, facilitating the development of drug reagents.
In addition to its natural nanobody library, NBLST also offers an off-the-shelf immunized library to help clients quickly screen for antibody molecules that meet their needs.
If you require our services, please feel free to contact us via email: marketingdept@nanobodylife.com







