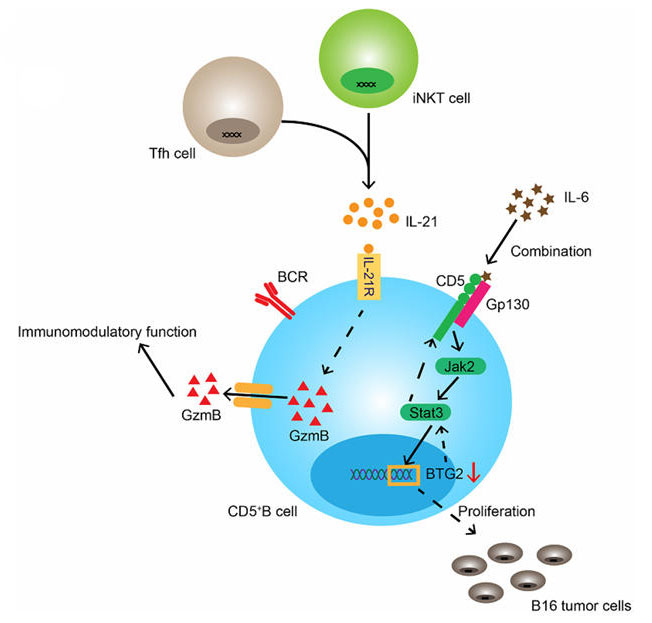
Mechanism of Action of the CD5 Target in Immune B Cells[1]
Clinical Application Areas for CD5 Antibodies
CD5 is fundamentally a key inhibitory co-receptor with a core function in modulating immune responses. When a T cell is activated by an antigen, the inhibitory signal transmitted by CD5 raises its activation threshold, preventing the immune system from overreacting to weak stimuli or self-antigens. However, this regulatory mechanism reveals its dual nature in disease progression. For example, in the tumor microenvironment of acute T-lymphoblastic leukemia (T-ALL) and T-cell lymphoma, tumor cells often highly and specifically express CD5 and may exploit its inhibitory signals to promote their own survival. Conversely, in autoimmune diseases such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA), CD5⁺ B cells exhibit a dual functionality: they can produce autoantibodies causing pathological damage, yet also secrete anti-inflammatory factors to exert a protective effect. This unique mechanism underpins the primary focus of CD5-targeted therapies and antibody drugs in two major clinical directions: hematological malignancies and autoimmune diseases.
Progress in CD5 Antibody Drug Development and Examples of Successful Drugs
Currently, the global development of CD5 antibody drugs is dominated by cellular immunotherapies, with several candidates having entered clinical stages and demonstrating significant therapeutic potential.
- CAR-T Cell Immunotherapy:
- MB-105:Developed by March Biosciences, MB-105 is arguably the most representative cellular immunotherapy drug at present. It has received Orphan Drug Designation (ODD) and Regenerative Medicine Advanced Therapy (RMAT) designation from the U.S. FDA for the treatment of relapsed/refractory CD5-positive T-cell lymphoma. Its core mechanism involves genetically engineering a patient's autologous T cells to express a chimeric antigen receptor (CAR) specifically recognizing CD5. Upon infusion, these CAR-T cells precisely bind to CD5-positive tumor cells, activate the T cell's killing signaling pathways, release cytotoxic substances like perforin and granzymes, and specifically lyse the tumor cells. The therapy's optimized CAR structure aims to reduce off-target risks, and it has shown promising tumor-killing activity and manageable safety in clinical settings.
- CT125:CT125 is a fully humanized, dual-epitope targeting CD5 CAR-T cell therapy product independently developed by IASO Bio. Its core mechanism involves using CRISPR/Cas9 technology to knock out the CD5 gene in the T cells themselves, fundamentally preventing issues of self-activation and fratricide that can occur when CAR-T cells express CD5. The CAR structure of CT125 enhances binding affinity to the CD5 antigen on tumor cells, improves T cell activation efficiency and tumor-killing specificity, enabling precise and efficient clearance of CD5-positive hematological tumor cells.
- VIPER-101:This drug is an autologous CAR-T cell immunotherapy product targeting CD5, developed by Vittoria Biotherapeutics based on their Senza5^TM^ platform. It uses gene editing to knock out the CD5 gene on the T cell surface, preventing mutual killing among CAR-T cells and ensuring their survival and proliferative capacity in vivo. Simultaneously, its CAR structure specifically binds to CD5 on tumor cells, activating T cell immune responses and releasing cytotoxic factors to eliminate the tumor. Currently, this drug has received clinical approval from the U.S. FDA for the treatment of relapsed or refractory T-cell lymphoma.
- Dual-Target/Enhanced Cellular Immunotherapies:
- AB-205:AB-205 is a CAR-NK cell immunotherapy product targeting CD5, co-developed through a collaboration between Artiva Biotherapeutics and GC Cell. The product incorporates a CAR with a CD5 antigen recognition domain and expresses soluble IL-15. After infusion, it can precisely recognize CD5^+^ T cells, exhibit specific cytotoxicity against leukemia cell lines CCRF-CEM and RPMI-8402, and leverages the natural absence or low expression of CD5 on NK cells to reduce the risk of fratricide against normal cells.
- ICG124:Developed by Icell Gene Therapeutics, ICG124 is an IL-15-expressing CD5-targeted CAR-T cell therapy product. This product incorporates an IL-15 self-expression domain into the traditional CAR structure. This composite CAR structure activates the T cell's tumor-killing activity upon binding to the CD5 antigen on tumor cells. Concurrently, the IL-15 secreted by this structure enhances T cell proliferation, survival, and cytotoxicity, improves the immunosuppressive state of the tumor microenvironment, achieving a dual effect of targeted killing and immune enhancement.
- BAH246(EB-BH2026):Developed by Essen Bio, this is a CD5/CD7 dual-target CAR-T cell therapy product. Its CAR structure can simultaneously express specific binding domains for both CD5 and CD7, enabling dual recognition of CD5 and CD7 antigens on the surface of acute lymphoblastic leukemia cells. This enhances tumor recognition specificity, reduces antigen escape and drug resistance, and activates T cell killing signals, boosting the level of activation and sustained tumor-killing capability.
Advantages of Nanobodies in CD5 Antibody Drug Development
Nanobodies have demonstrated remarkable performance in drug development research for various target molecules due to their exceptional penetrability, high stability, and ease of engineering. For example, their small molecular volume allows them to easily penetrate to the core of the tumor microenvironment, achieving high-concentration targeted drug delivery. Furthermore, their ease of engineering enables their use as targeted delivery vehicles to enhance immune activation functions, prolong in vivo half-life, while also potentially avoiding off-target toxicity and fratricide phenomena.
For instance, in a study jointly conducted by institutions including Guangdong Provincial People's Hospital and Southern Medical University, a drug molecule named BiCD30/5-GF was designed. This molecule consists of nanobodies specifically targeting CD5 and CD30 linked in tandem, conjugated via a Gv/Sd system to human ferritin (h-HFn) and granzyme B (GrB). Utilizing dual CD5/CD30 targeting to recognize T-cell lymphoma cells and exploiting the binding of h-HFn to CD71 to enhance cellular internalization, it can precisely identify tumor cells and efficiently mediate intracellular GrB delivery, activating the caspase-3/BID pathway to induce apoptosis[2].
Another study, led by West China Hospital of Sichuan University, developed 1H6-15-NKCE, a trispecific structure using an anti-CD5 single-chain antibody as the targeting module, paired with an anti-CD16a nanobody, and fused with an IL-15Rα/IL-15 complex. It achieves specific lysis of tumor cells through a triple mechanism: CD5 targeting to bind tumor cells, CD16a engaging NK cells (enhancing binding), and IL-15 stimulating the JAK/STAT signaling pathway to enhance NK cell proliferation and activity[3].
Both studies showcase the characteristics of nanobodies: high specificity, low off-target risk, precise recognition of antigen epitopes, strong penetration into the tumor microenvironment, and high delivery efficiency. Additionally, due to their flexible structure and ease of multi-module modification, nanobodies can be adapted to different therapeutic scenarios. After humanization, they demonstrate unique advantages such as long half-life, low immunogenicity, and potentially superior safety.
NBLST possesses pDual phage display technology. Building upon the efficient development of traditional phage display, this technology seamlessly connects to efficient mammalian cell production, significantly improving the efficiency of screening out problematic molecules. Furthermore, the NabLib® mammalian cell display technology not only enhances the developability of screened molecules but also allows flexible selection of antibody screening formats, providing better assurance for downstream antibody molecule application and detection.
Concurrently, Nabo Life offers an off-the-shelf CD5 immune library. After alpaca immunization, we collect whole blood, isolate PBMCs, and cryopreserve them in the form of a cell bank. Clients can bypass the lengthy immunization period and proceed directly to the screening process, greatly saving time in antibody molecule development. Through customized screening services, multiple antibody molecules that best meet application requirements can be obtained.